
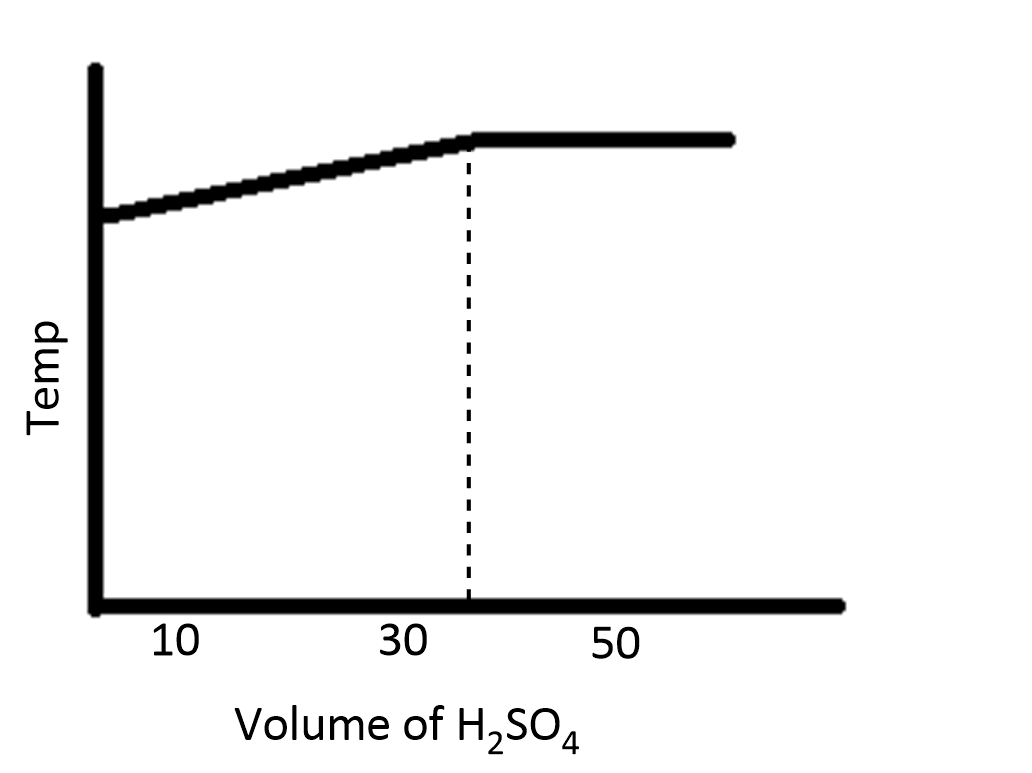
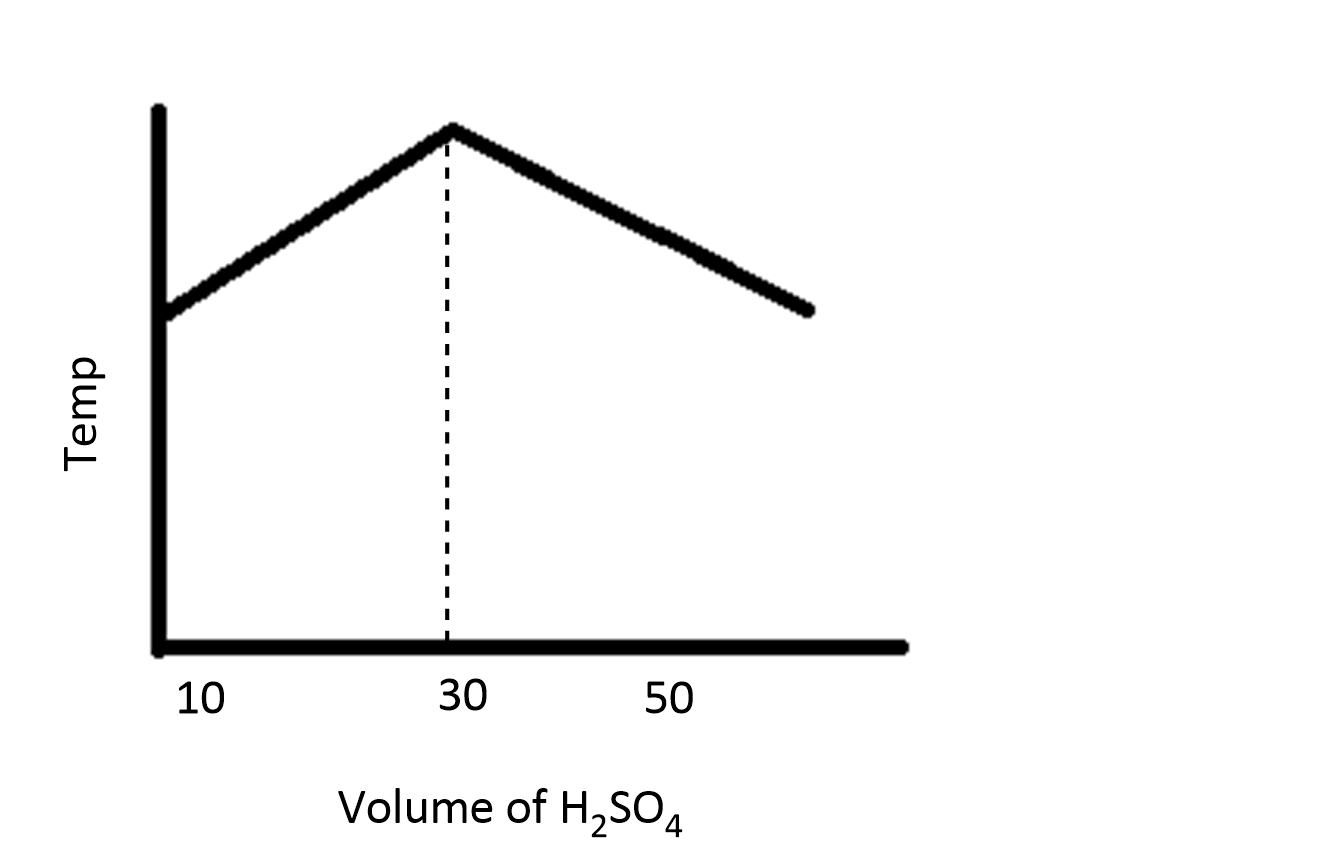
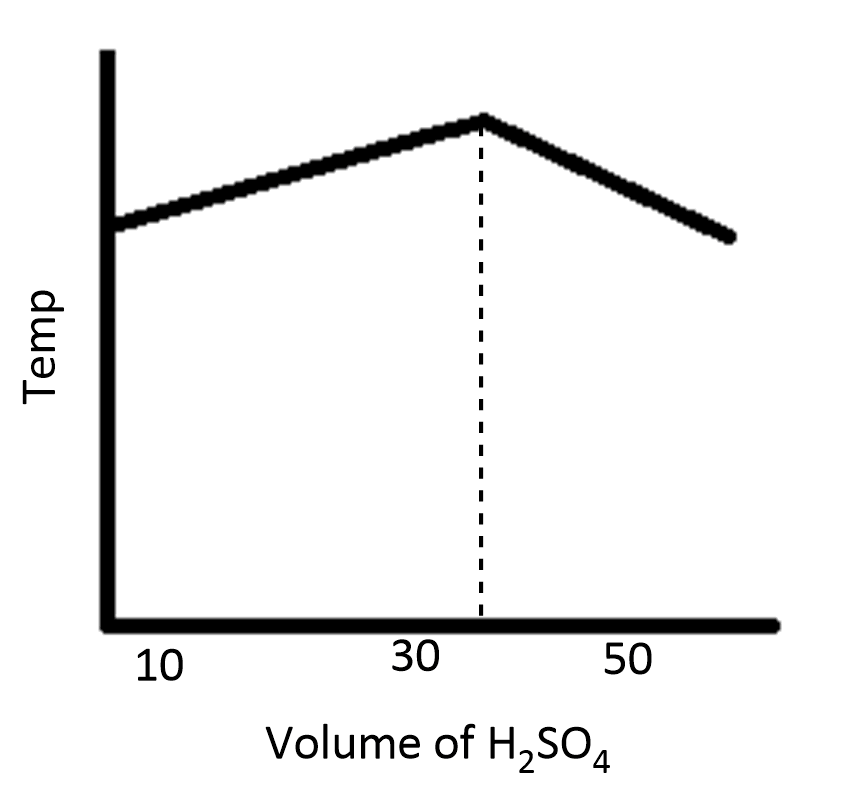
In an experiment to determine the enthalpy of neutralization of sodium hydroxide with sulfuric acid, $50c{m^3}$ of $0.4M$ sodium hydroxide were titrated thermometrically with $0.25M$ sulfuric acid. Which of the following plots gives the correct representation?
A.

B.
C.
D.




Answer
563.1k+ views
Hint: We can predict the correct representation by calculating the volume of the solution using the normality and the milliequivalents. Initially, we have to calculate the volume of the sulfuric acid solution by dividing the number of milliequivalents to the normality of the solution.
Complete step by step answer:
Volume of sodium hydroxide is $50c{m^3}$
Molarity of sodium hydroxide is $0.4M$
Molarity of sulfuric acid is $0.25M$
We have to know the number of milliequivalents of sulfuric acid.
So, the number of milliequivalents of sulfuric acid required for 20Meq of sodium hydroxide is twenty.
Let us consider the required volume of sulfuric acid to be V mL.
The volume of ${H_2}S{O_4}$ is calculated using the milliequivalents and volume. The product of normality and milliequivalents will be the volume.
We can write the formula as,
${\text{Number of milliequivalents}} = {\text{Normality}} \times {\text{Volume}}$
We know that the basicity of sulfuric acid is two.
So, we can calculate the volume by rearranging the equation of the number of milliequivalents.
${\text{Volume}} = \dfrac{{{\text{Number of milliequivalents}}}}{{{\text{Normality}}}}$
Let us now substitute the values of the number of mill equivalents and normality.
${\text{Volume}} = \dfrac{{{\text{Number of milliequivalents}}}}{{{\text{Normality}}}}$
On substituting the known values we get,
\[{\text{Volume}} = \dfrac{{20}}{{0.25 \times 2}}\]
\[{\text{Volume}} = \dfrac{{20}}{{0.5}}\]
On simplification we get,
\[{\text{Volume}} = 40mL\]
The volume of the solution is 40mL.
During the process of neutralization, the temperature increases and then decreases because of the increase in volume of the solution.
Therefore, the option D is correct.
Note: We have to know that Enthalpy of neutralization is always fixed in case of strong acid and a strong base: this is because strong acids and strong bases are fully ionized in dilute solution. The changes of enthalpy in neutralization are negative for a reaction of acid and base, and in this process heat is liberated out. In the case of weak acids or bases, the heat of neutralization is dependent on pH. In the absence of any added mineral acid or alkali a few amounts of heat is needed for complete dissociation to occur. The total heat evolved during the process neutralization would be smaller.
Complete step by step answer:
Volume of sodium hydroxide is $50c{m^3}$
Molarity of sodium hydroxide is $0.4M$
Molarity of sulfuric acid is $0.25M$
We have to know the number of milliequivalents of sulfuric acid.
So, the number of milliequivalents of sulfuric acid required for 20Meq of sodium hydroxide is twenty.
Let us consider the required volume of sulfuric acid to be V mL.
The volume of ${H_2}S{O_4}$ is calculated using the milliequivalents and volume. The product of normality and milliequivalents will be the volume.
We can write the formula as,
${\text{Number of milliequivalents}} = {\text{Normality}} \times {\text{Volume}}$
We know that the basicity of sulfuric acid is two.
So, we can calculate the volume by rearranging the equation of the number of milliequivalents.
${\text{Volume}} = \dfrac{{{\text{Number of milliequivalents}}}}{{{\text{Normality}}}}$
Let us now substitute the values of the number of mill equivalents and normality.
${\text{Volume}} = \dfrac{{{\text{Number of milliequivalents}}}}{{{\text{Normality}}}}$
On substituting the known values we get,
\[{\text{Volume}} = \dfrac{{20}}{{0.25 \times 2}}\]
\[{\text{Volume}} = \dfrac{{20}}{{0.5}}\]
On simplification we get,
\[{\text{Volume}} = 40mL\]
The volume of the solution is 40mL.
During the process of neutralization, the temperature increases and then decreases because of the increase in volume of the solution.
Therefore, the option D is correct.
Note: We have to know that Enthalpy of neutralization is always fixed in case of strong acid and a strong base: this is because strong acids and strong bases are fully ionized in dilute solution. The changes of enthalpy in neutralization are negative for a reaction of acid and base, and in this process heat is liberated out. In the case of weak acids or bases, the heat of neutralization is dependent on pH. In the absence of any added mineral acid or alkali a few amounts of heat is needed for complete dissociation to occur. The total heat evolved during the process neutralization would be smaller.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




