
In allene (${C_3}{H_4}$), the type(s) of hybridisation of the carbon atoms is(are):
A) $sp$ and $s{p^3}$
B) $sp$ and $s{p^2}$
C) Only $s{p^2}$
D) $s{p^2}$ and $s{p^3}$
Answer
579.9k+ views
Hint: First you need to draw the chemical structure of allene. In allenes, each carbon atom has double bonds with its adjacent carbon centres. Allenes are cumulated dienes. The central carbon in allene forms two sigma bonds and two pi bonds, so think about its hybridisation.
Complete step by step answer:
Allenes are the organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres. The parent compound of the class of allenes is propadiene, ${C_3}{H_4}$, which is itself also called allene and is the simplest allene. The structure of allene (${C_3}{H_4}$) is as follows:
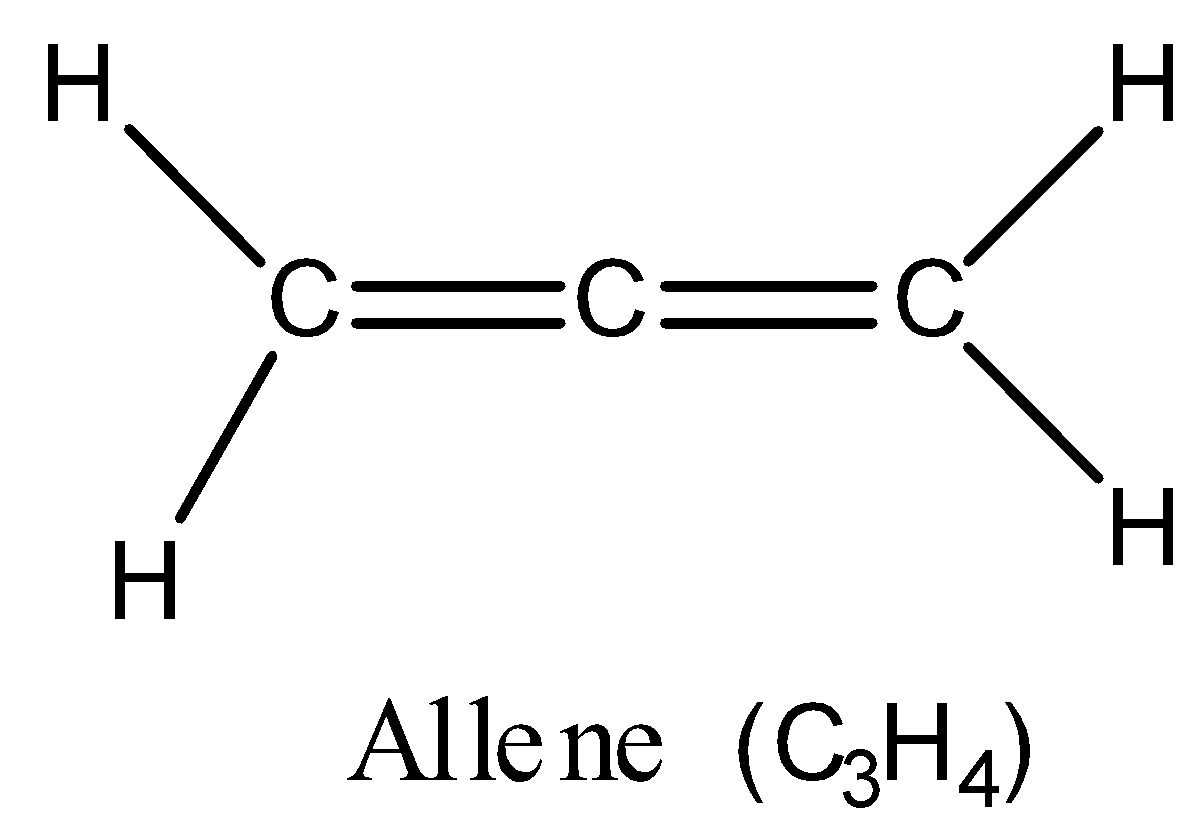
Now, let us come to the structure and bonding in the allene.
Valence shell electronic configuration of carbon: $2{s^2}2{p^2}$
You can see in the above structure that the central carbon atom in the allene forms two double bonds in which there are two sigma bonds and two pi bonds. When there is an equal amount of s and p-character in the bonding hybridised orbital, then hybridisation is $sp$. Hence, hybridisation of the central carbon atom in allene is $sp$.
There are two terminal carbon atoms in the allene structure. The terminal carbon atoms are linked to two hydrogen atoms by single covalent bonds and to one adjacent carbon atom by a double bond. Thus, there is involvement of one s-orbital and two p-orbitals in the bonding of the terminal carbon atoms. Hence, hybridisation of the two terminal carbon atoms in allene is $s{p^2}$.
Therefore, in allene (${C_3}{H_4}$), the types of hybridisation of the carbon atoms are $sp$ and $s{p^2}$.
So, the correct answer is “Option B”.
Note: The double bonds in allenes do not exhibit resonance. The compounds with an allene type structure but having more than three carbon atoms are members of a larger class of compounds known as cumulenes with $X = C = Y$ bonding type.
Complete step by step answer:
Allenes are the organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres. The parent compound of the class of allenes is propadiene, ${C_3}{H_4}$, which is itself also called allene and is the simplest allene. The structure of allene (${C_3}{H_4}$) is as follows:

Now, let us come to the structure and bonding in the allene.
Valence shell electronic configuration of carbon: $2{s^2}2{p^2}$
You can see in the above structure that the central carbon atom in the allene forms two double bonds in which there are two sigma bonds and two pi bonds. When there is an equal amount of s and p-character in the bonding hybridised orbital, then hybridisation is $sp$. Hence, hybridisation of the central carbon atom in allene is $sp$.
There are two terminal carbon atoms in the allene structure. The terminal carbon atoms are linked to two hydrogen atoms by single covalent bonds and to one adjacent carbon atom by a double bond. Thus, there is involvement of one s-orbital and two p-orbitals in the bonding of the terminal carbon atoms. Hence, hybridisation of the two terminal carbon atoms in allene is $s{p^2}$.
Therefore, in allene (${C_3}{H_4}$), the types of hybridisation of the carbon atoms are $sp$ and $s{p^2}$.
So, the correct answer is “Option B”.
Note: The double bonds in allenes do not exhibit resonance. The compounds with an allene type structure but having more than three carbon atoms are members of a larger class of compounds known as cumulenes with $X = C = Y$ bonding type.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




