
In a simple cubic lattice with a unit cell length equal to 0.5nm, a lattice plane is parallel to the y-axis and has interference in the ratio of 2:3 along the y and z axis. Calculate the miller indices and sketch the lane. Also calculate the interplanar distance, x-rays of wavelength which are incident on these planes. Calculate the Bragg’s equation for second order diffraction.
Answer
570k+ views
Hint: We must remember that all the crystal structures have a basic cubic shape; the difference lies within the way atoms are arranged in them. All lattice has atoms present at the vertices of the cubic unit. In addition: in simple cubic unit, the only one atom involved are those at the vertices
Complete answer:
Let’s understand from the question. Here we’ve given:
Length of unit cell=0.5nm
Lattice plane is parallel to y-axis
The line intercept at y-axis is infinity
Intercept ratio of x: z is 2:3
Intercept along x, y and z are 2: infinity: 3
Now we take reciprocal on intercept = \[\dfrac{1}{2}:\dfrac{1}{\infty }:\dfrac{1}{3}\]
Now we multiply above equation with 6 = 3: 0: 2
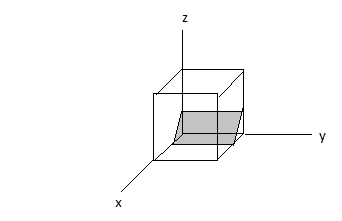
Miller indices (x, y, z) = (3, 0, 2)
Now we will calculate interplanar spacing (d) = \[\dfrac{a}{{\sqrt {{x^2} + {y^2} + {z^2}} }}\]
Where,
a = unit cell length
Now we can substitute the known values we get,
$\Rightarrow$ d=\[\dfrac{{0.5}}{{\sqrt {{3^2} + 0 + {2^2}} }}\]
$\Rightarrow$ d\[ = \dfrac{{0.5}}{{\sqrt {13} }}\]
On simplification we get,
$d = 1.38A^\circ $
Wavelength (\[\lambda ) = \sin \theta \dfrac{{2a}}{{\sqrt {{x^2} + {y^2} + {z^2}} }}\]
$\Rightarrow$ \[ = \sin \theta \dfrac{{2 \times 0.5}}{{\sqrt {13} }} = 0\]
From, Bragg’s equation
\[n\lambda = 2d\sin \theta \]
Where,
n=Integer
λ= wavelength of light passed
d=distance between the crystal planes
θ=angle of incidence
Where, n = 2, d= 1.38 A, \[\lambda = 0\]
\[\theta = {\sin ^{ - 1}}\left( {\dfrac{\lambda }{d}} \right) \times n\]
Substituting the known values we get,
\[ \Rightarrow \theta = {\sin ^{ - 1}}\dfrac{0}{{1.38}} \times 2 = 0\]
Note:
We have to remember that in simple cubic unit cell atoms are present only at corners. It's a primitive unit cell. In other unknit cells atoms are present at corners also as at other positions. In body centred cubic lattice atoms are present at corners also because the centre of the cube, in face centred cubic system atoms are present at corners also as at the centre of every face of the cube.
Complete answer:
Let’s understand from the question. Here we’ve given:
Length of unit cell=0.5nm
Lattice plane is parallel to y-axis
The line intercept at y-axis is infinity
Intercept ratio of x: z is 2:3
Intercept along x, y and z are 2: infinity: 3
Now we take reciprocal on intercept = \[\dfrac{1}{2}:\dfrac{1}{\infty }:\dfrac{1}{3}\]
Now we multiply above equation with 6 = 3: 0: 2

Miller indices (x, y, z) = (3, 0, 2)
Now we will calculate interplanar spacing (d) = \[\dfrac{a}{{\sqrt {{x^2} + {y^2} + {z^2}} }}\]
Where,
a = unit cell length
Now we can substitute the known values we get,
$\Rightarrow$ d=\[\dfrac{{0.5}}{{\sqrt {{3^2} + 0 + {2^2}} }}\]
$\Rightarrow$ d\[ = \dfrac{{0.5}}{{\sqrt {13} }}\]
On simplification we get,
$d = 1.38A^\circ $
Wavelength (\[\lambda ) = \sin \theta \dfrac{{2a}}{{\sqrt {{x^2} + {y^2} + {z^2}} }}\]
$\Rightarrow$ \[ = \sin \theta \dfrac{{2 \times 0.5}}{{\sqrt {13} }} = 0\]
From, Bragg’s equation
\[n\lambda = 2d\sin \theta \]
Where,
n=Integer
λ= wavelength of light passed
d=distance between the crystal planes
θ=angle of incidence
Where, n = 2, d= 1.38 A, \[\lambda = 0\]
\[\theta = {\sin ^{ - 1}}\left( {\dfrac{\lambda }{d}} \right) \times n\]
Substituting the known values we get,
\[ \Rightarrow \theta = {\sin ^{ - 1}}\dfrac{0}{{1.38}} \times 2 = 0\]
Note:
We have to remember that in simple cubic unit cell atoms are present only at corners. It's a primitive unit cell. In other unknit cells atoms are present at corners also as at other positions. In body centred cubic lattice atoms are present at corners also because the centre of the cube, in face centred cubic system atoms are present at corners also as at the centre of every face of the cube.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




