
If the following compound is treated with Pd/C in excess of hydrogen gas, how many stereoisomers of the product will be obtained?
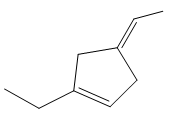
(A) 1
(B) 2
(C) 3
(D) 4

Answer
583.2k+ views
Hint: Palladium over carbon in excess of hydrogen gas catalyzes reduction reaction and carbon carbon double bond are reduced to carbon carbon single bond. Number of stereoisomers have the following formula= ${{2}^{n}}$ where n indicates number of chiral carbon atoms and chiral carbon is carbon atom attached to four different groups or atoms.
Complete solution step by step:
Palladium over carbon catalyzes in excess of hydrogen gas catalyzes reduction reaction and carbon carbon double bond is reduced to carbon carbon single bond. So, unsaturated compounds are converted to saturated compounds.
Chiral carbon is a carbon atom attached to four different atoms or groups. In this carbon atom is asymmetric.
Stereoisomers are molecules having the same molecular formula but different arrangement of atoms in space.
Number of stereoisomers can be determined by using following formula: \[{{2}^{n}}\] where n is number of chiral carbon atoms
Molecules which have lack of symmetry or do not possess planes of symmetry, are chiral.
When a given molecule undergoes reduction, the following product is formed which has two chiral carbon atoms.
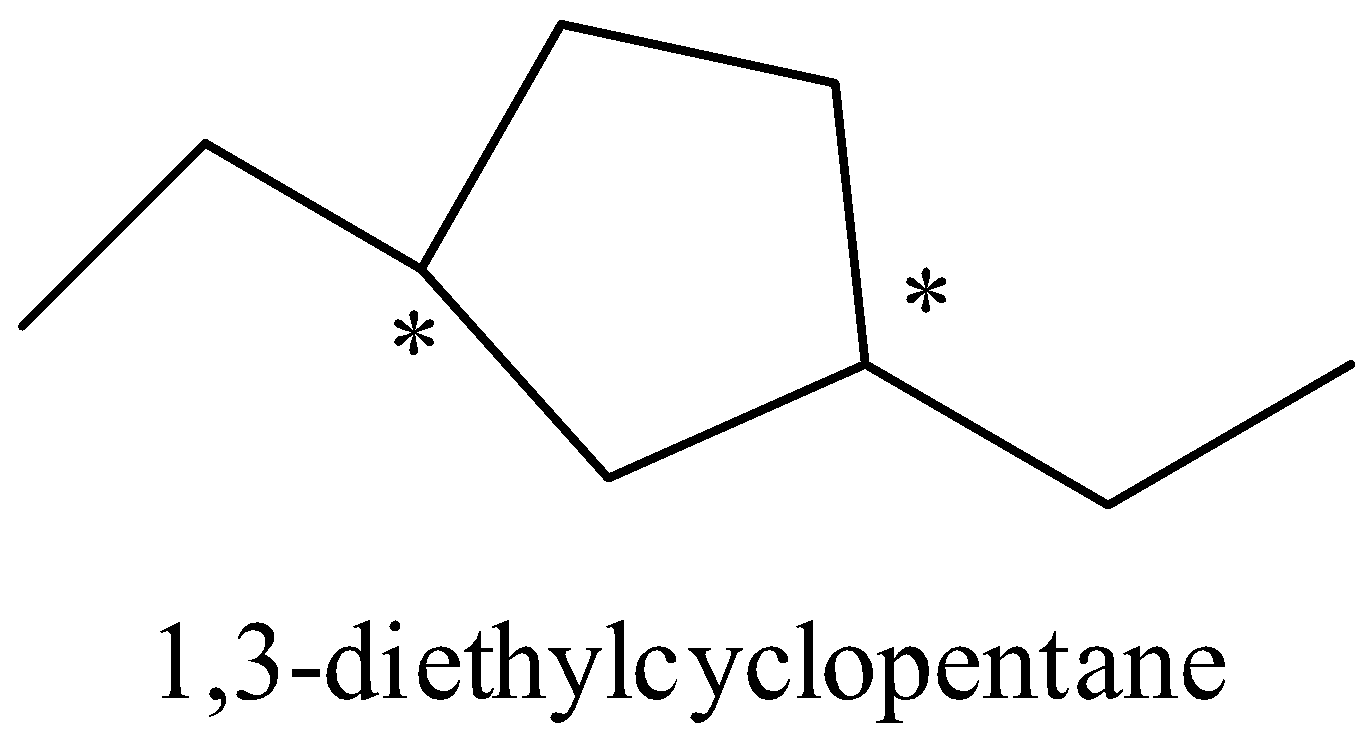
Number of stereoisomers for this structure can be calculated as there are two chiral carbon atom are=${{2}^{n}}={{2}^{2}}$=4
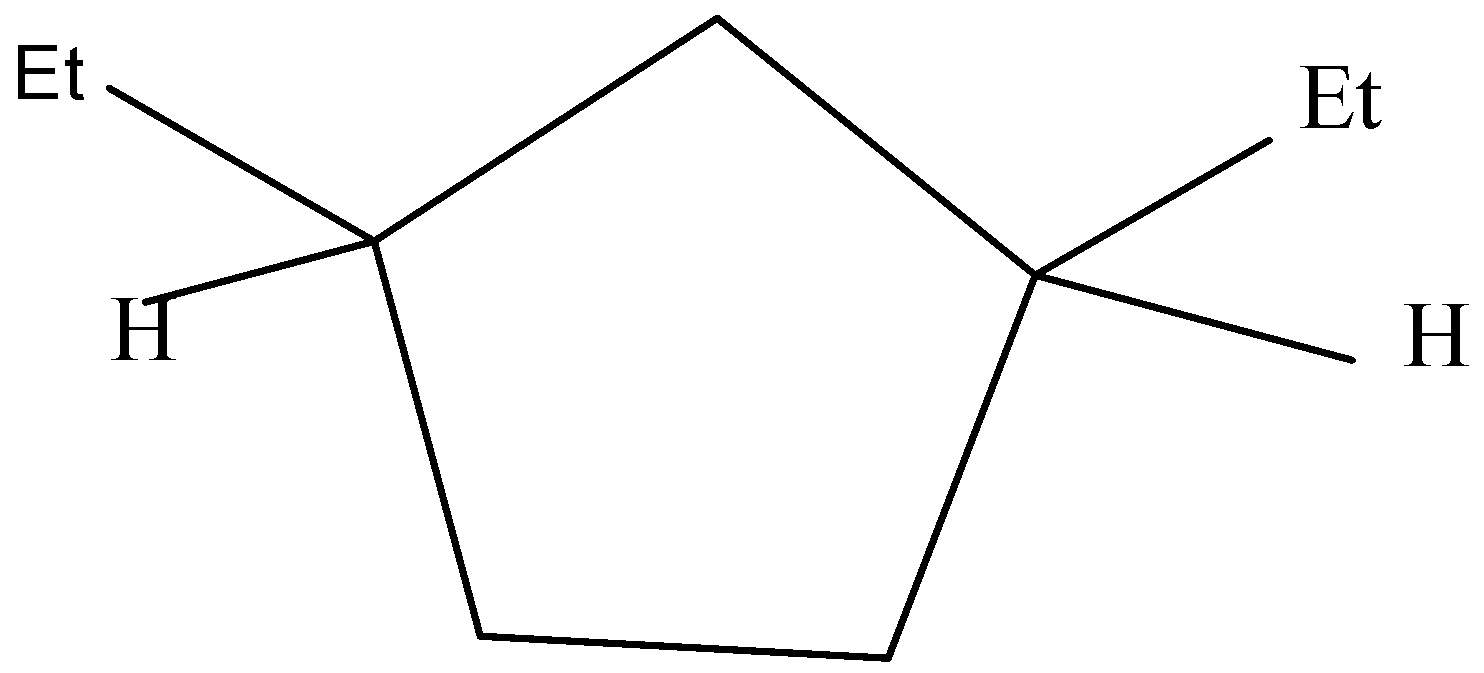
One of the stereoisomers is in meso form which means it has a plane of symmetry.
The following structure does not have a plane of symmetry and has a non-superimposable mirror image.
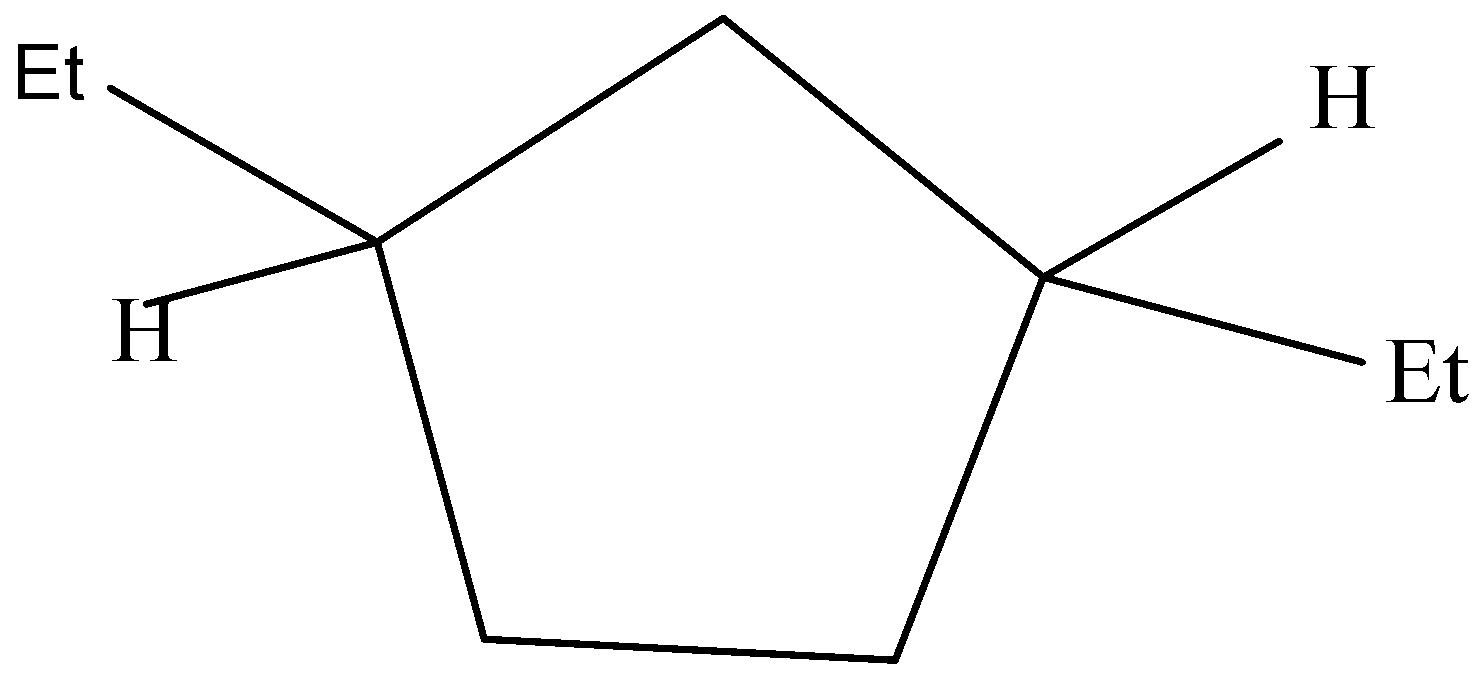
So, (C) 3 stereoisomers of the product will be obtained.
Note: Enantiomers are non-superimposable mirror images that have all properties same except the direction in which they rotate the plane of plane polarized light. Meso forms have a plane of symmetry, hence they do not possess chirality. Molecules which have lack of symmetry or do not possess planes of symmetry, are chiral.
Complete solution step by step:
Palladium over carbon catalyzes in excess of hydrogen gas catalyzes reduction reaction and carbon carbon double bond is reduced to carbon carbon single bond. So, unsaturated compounds are converted to saturated compounds.
Chiral carbon is a carbon atom attached to four different atoms or groups. In this carbon atom is asymmetric.
Stereoisomers are molecules having the same molecular formula but different arrangement of atoms in space.
Number of stereoisomers can be determined by using following formula: \[{{2}^{n}}\] where n is number of chiral carbon atoms
Molecules which have lack of symmetry or do not possess planes of symmetry, are chiral.
When a given molecule undergoes reduction, the following product is formed which has two chiral carbon atoms.

Number of stereoisomers for this structure can be calculated as there are two chiral carbon atom are=${{2}^{n}}={{2}^{2}}$=4
One of the stereoisomers is in meso form which means it has a plane of symmetry.

The following structure does not have a plane of symmetry and has a non-superimposable mirror image.

So, (C) 3 stereoisomers of the product will be obtained.
Note: Enantiomers are non-superimposable mirror images that have all properties same except the direction in which they rotate the plane of plane polarized light. Meso forms have a plane of symmetry, hence they do not possess chirality. Molecules which have lack of symmetry or do not possess planes of symmetry, are chiral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




