
If the distance between\[{\rm{N}}{{\rm{a}}^ + }\] and \[{\rm{C}}{{\rm{l}}^ - }\]ions in sodium chloride crystal is \[{\rm{X}}\,{\rm{pm}}\], the length of the edge of the unit cell is:
(A) \[{\rm{4X}}\,{\rm{pm}}\]
(B) \[\dfrac{{\rm{X}}}{{\rm{4}}}\,{\rm{pm}}\]
(C) \[\dfrac{{\rm{X}}}{2}\,{\rm{pm}}\]
(D) \[{\rm{2X}}\,{\rm{pm}}\]
Answer
566.7k+ views
Hint: As we know that, the face centred cubic lattice is a unit cell in which number of atoms are present at eight corners as well as at the face of the body. Distance between charged ions in the lattice can be calculated by calculating the radius of atom known as the edge of the lattice or lattice parameter.
Step by step answer: sodium chloride is also known as rack salt structure and it follows FCC cubic lattice in which \[{\rm{N}}{{\rm{a}}^ + }\]ions present in all the octahedral voids(\[{{\rm{O}}_{\rm{h}}}\]) and \[{\rm{C}}{{\rm{l}}^ - }\]in all the corners and face centre of the unit cell.
octahedral voids- present at body centre+ at each edge centre
FCC lattice-present at eight corners + face centre of the cubic unit cell
The structure of rack salt is shown below.
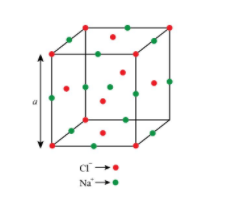
In the above structure, the edge is represented as (a).
Now, we are given as the distance between \[{\rm{N}}{{\rm{a}}^ + }\] and \[{\rm{C}}{{\rm{l}}^ - }\]ions in sodium chloride crystal is \[{\rm{X}}\,{\rm{pm}}\]So, from the above structure, it is clear that the edge length of the crystal is twice the distance between \[{\rm{N}}{{\rm{a}}^ + }\] and \[{\rm{C}}{{\rm{l}}^ - }\]ions. So, we can say that the length of the edge of the unit cell is \[{\rm{2X}}\,{\rm{pm}}\].
Therefore, the correct answer is option (D).
Note: The voids or holes are the empty spaces between the arrangements of the atoms in the crystal lattice. It tells us about the shape or structure of the void present inside any lattice structure There are two types of voids
(a) tetrahedral void
(b) octahedral void
Step by step answer: sodium chloride is also known as rack salt structure and it follows FCC cubic lattice in which \[{\rm{N}}{{\rm{a}}^ + }\]ions present in all the octahedral voids(\[{{\rm{O}}_{\rm{h}}}\]) and \[{\rm{C}}{{\rm{l}}^ - }\]in all the corners and face centre of the unit cell.
octahedral voids- present at body centre+ at each edge centre
FCC lattice-present at eight corners + face centre of the cubic unit cell
The structure of rack salt is shown below.

In the above structure, the edge is represented as (a).
Now, we are given as the distance between \[{\rm{N}}{{\rm{a}}^ + }\] and \[{\rm{C}}{{\rm{l}}^ - }\]ions in sodium chloride crystal is \[{\rm{X}}\,{\rm{pm}}\]So, from the above structure, it is clear that the edge length of the crystal is twice the distance between \[{\rm{N}}{{\rm{a}}^ + }\] and \[{\rm{C}}{{\rm{l}}^ - }\]ions. So, we can say that the length of the edge of the unit cell is \[{\rm{2X}}\,{\rm{pm}}\].
Therefore, the correct answer is option (D).
Note: The voids or holes are the empty spaces between the arrangements of the atoms in the crystal lattice. It tells us about the shape or structure of the void present inside any lattice structure There are two types of voids
(a) tetrahedral void
(b) octahedral void
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Why is there a time difference of about 5 hours between class 10 social science CBSE

Who Won 36 Oscar Awards? Record Holder Revealed

What is the median of the first 10 natural numbers class 10 maths CBSE

Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

Who is the executive head of the government APresident class 10 social science CBSE




