
If \[a\] be the edge length of the unit cell and $r$ be the radius of an atom then for $fcc$ arrangement, the correct relation is:
A. $4a = \sqrt 3 r$
B. $4r = \sqrt 3 a$
C. $4r = \sqrt 2 a$
D. $4r = \dfrac{a}{{\sqrt 2 }}$
Answer
564.9k+ views
Hint:The structure of $fcc$ arrangement is specific because the representation has a specific geometry. Each of the lengths is similar as the structure is the same as that of a cube. The location where the atoms are present is defined by the nature of the lattice.
Complete step by step answer:
According to the given condition each of the faces of the cube structure has a particular atom at the centre. This is why it is known as $FCC$ or face-centred lattice. Each of the edges or the side of the lattice are similar in length and each side can be considered $a$. Since there is an atom at the middle of each of the face and atoms on the edges, the diagrammatic representation would look like this:
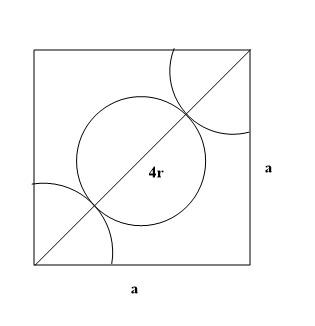
According to the given picture it can be proved that for defining the diagonals on each side, the representation can be done with the help of
${r_f} = r + 2r + r$
$ \Rightarrow {r_f} = 4r$
Here ${r_f}$ is the diagonal which is seen to be four times that of the radius of each atom according to representation in the diagram. The two sides are $a$ which are part of a single face. Therefore, the whole structure can be depicted as a right-angled triangle. According to Pythagoras theorem, the representation of this triangle will be:
${a^2} + {a^2} = {\left( {4r} \right)^2}$
$ \Rightarrow 2{a^2} = {\left( {4r} \right)^2}$
$ \Rightarrow \sqrt 2 a = 4r$ (After taking square root on both sides)
According to conditions of $FCC$ lattice the relation between radius of each atom and each side is: C.$\sqrt 2 a = 4r$.
Note:
The relation between the variables of the given structure can be represented properly among all the lattices. The condition differs among the different types of lattice structures based on the presence of specific atoms in specific regions. The difference in specific structure is based on types of atoms involved in the process.
Complete step by step answer:
According to the given condition each of the faces of the cube structure has a particular atom at the centre. This is why it is known as $FCC$ or face-centred lattice. Each of the edges or the side of the lattice are similar in length and each side can be considered $a$. Since there is an atom at the middle of each of the face and atoms on the edges, the diagrammatic representation would look like this:

According to the given picture it can be proved that for defining the diagonals on each side, the representation can be done with the help of
${r_f} = r + 2r + r$
$ \Rightarrow {r_f} = 4r$
Here ${r_f}$ is the diagonal which is seen to be four times that of the radius of each atom according to representation in the diagram. The two sides are $a$ which are part of a single face. Therefore, the whole structure can be depicted as a right-angled triangle. According to Pythagoras theorem, the representation of this triangle will be:
${a^2} + {a^2} = {\left( {4r} \right)^2}$
$ \Rightarrow 2{a^2} = {\left( {4r} \right)^2}$
$ \Rightarrow \sqrt 2 a = 4r$ (After taking square root on both sides)
According to conditions of $FCC$ lattice the relation between radius of each atom and each side is: C.$\sqrt 2 a = 4r$.
Note:
The relation between the variables of the given structure can be represented properly among all the lattices. The condition differs among the different types of lattice structures based on the presence of specific atoms in specific regions. The difference in specific structure is based on types of atoms involved in the process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




