
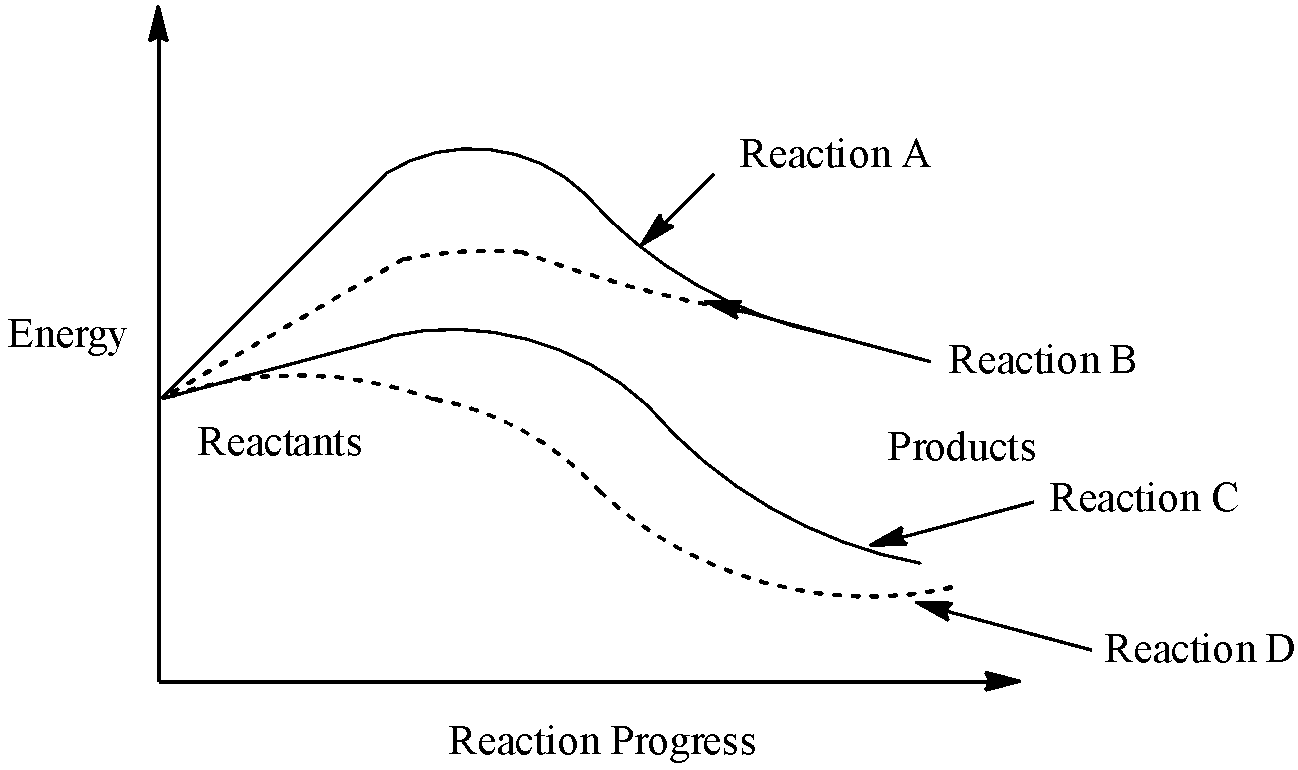
Identify the type of reaction indicated by D in the diagram.
(a) Uncatalyzed exothermic
(b) catalyzed exothermic
(c) catalyzed exothermic
(d) uncatalyzed endothermic

Answer
554.4k+ views
Hint: The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. The activation energy of the uncatalyzed reaction and catalysed reaction is shown by ${{E}_{a}}$. The heat of reaction ($\Delta H$) is unchanged by the presence of the catalyst. The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalysed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy.
Complete answer:
We have been provided with a diagram which shows:
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve,
We need to tell what is indicated by D in the above diagram,
So, for that:
Uncatalyzed endothermic reaction: It is a reaction in which, it requires a maximum activation energy for initiating the reaction. Also, it is an endothermic reaction as the energy of the product is greater than the energy of the reactant.
The overall exothermic or endothermic energy change is the same for both the catalysed or uncatalyzed reaction. The catalyst might help break the bonds BUT it cannot change the actual bond energies.
On analysing the given figure carefully, it can be easily concluded that:
A – Uncatalyzed endothermic reaction.
B – Catalysed endothermic reaction.
C – Catalysed exothermic reaction.
A is an uncatalyzed reaction because it requires a maximum activation energy for initiating the reaction.
C represents an uncatalyzed exothermic reaction as the energy of products is less than that of reactants.
D represents catalysed exothermic reaction as its final energy is same as that of C but it took a low energy pathway for the completion of the reaction.
So, we can say that in the figure catalysed exothermic reaction is indicated by D.
Therefore, option (b) is correct.
Note: A potential energy diagram shows the change in energy during a reaction. Heat of Reaction, $\Delta H$: the overall difference in potential energy between the products and the reactants. In an endothermic reaction, the energy of the products is greater than the energy of the reactants and $\Delta H$ is positive.
Complete answer:
We have been provided with a diagram which shows:
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve,
We need to tell what is indicated by D in the above diagram,
So, for that:
Uncatalyzed endothermic reaction: It is a reaction in which, it requires a maximum activation energy for initiating the reaction. Also, it is an endothermic reaction as the energy of the product is greater than the energy of the reactant.
The overall exothermic or endothermic energy change is the same for both the catalysed or uncatalyzed reaction. The catalyst might help break the bonds BUT it cannot change the actual bond energies.
On analysing the given figure carefully, it can be easily concluded that:
A – Uncatalyzed endothermic reaction.
B – Catalysed endothermic reaction.
C – Catalysed exothermic reaction.
A is an uncatalyzed reaction because it requires a maximum activation energy for initiating the reaction.
C represents an uncatalyzed exothermic reaction as the energy of products is less than that of reactants.
D represents catalysed exothermic reaction as its final energy is same as that of C but it took a low energy pathway for the completion of the reaction.
So, we can say that in the figure catalysed exothermic reaction is indicated by D.
Therefore, option (b) is correct.
Note: A potential energy diagram shows the change in energy during a reaction. Heat of Reaction, $\Delta H$: the overall difference in potential energy between the products and the reactants. In an endothermic reaction, the energy of the products is greater than the energy of the reactants and $\Delta H$ is positive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




