
Identify the most reactive element of group IA.
Answer
598.5k+ views
Hint: The number of electrons in the outermost shell of an atom and its tendency to lose electrons determines its reactivity. For example, Noble gases have low reactivity because they have full electron shells
Complete step by step answer:
1. Group IA is also called the alkali group which includes Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr).
2. The group IA elements are generally soft, reactive metals with low melting points. They easily react with water to form an alkaline metal hydroxide solution and hydrogen. Reactivity of IA elements increases down the group.
3. Alkali metals are among the most reactive metals in the periodic table and this is due to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and have an oxidation state of +1.
4. As we know that reactivity increases down the group, So, you must be thinking that Francium is looking more reactive. But it is not the case, Francium has a heavier nucleus than Cesium and it causes radioactivity. Due to that heavy nuclei the interactions between the nucleus and the electron is more and hence it requires high energy to remove an electron from the outermost shell. There is no relation of radioactivity and tendency to forming compounds. Down to the Francium the reactivity must be slightly decreased due to the heavy nuclei.
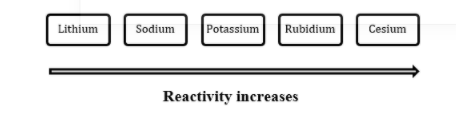
So, Cesium is the most reactive element in group IA.
Note: All the alkali metals react vigorously with halogens to produce salts, as the halogens needs one electron to complete its octet and alkali metals can readily lose electrons to have an oxidation state of +1.
Complete step by step answer:
1. Group IA is also called the alkali group which includes Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr).
2. The group IA elements are generally soft, reactive metals with low melting points. They easily react with water to form an alkaline metal hydroxide solution and hydrogen. Reactivity of IA elements increases down the group.
3. Alkali metals are among the most reactive metals in the periodic table and this is due to their larger atomic radii and low ionization energies. They tend to donate their electrons in reactions and have an oxidation state of +1.
4. As we know that reactivity increases down the group, So, you must be thinking that Francium is looking more reactive. But it is not the case, Francium has a heavier nucleus than Cesium and it causes radioactivity. Due to that heavy nuclei the interactions between the nucleus and the electron is more and hence it requires high energy to remove an electron from the outermost shell. There is no relation of radioactivity and tendency to forming compounds. Down to the Francium the reactivity must be slightly decreased due to the heavy nuclei.

So, Cesium is the most reactive element in group IA.
Note: All the alkali metals react vigorously with halogens to produce salts, as the halogens needs one electron to complete its octet and alkali metals can readily lose electrons to have an oxidation state of +1.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




