
Identify the hydrophilic and hydrophobic parts in the following non-ionic detergent present in liquid detergents and wetting agents.
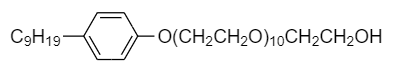
A. Hydrophobic part: $ - C{H_2}C{H_2}OH $
Hydrophilic part:
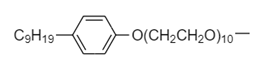
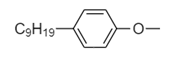
B. Hydrophobic part:
Hydrophilic part: $ - O{(C{H_2}C{H_2}O)_{10}}C{H_2}C{H_2}OH $
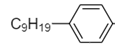
C. Hydrophobic part:
Hydrophilic part: $ - {(C{H_2}C{H_2}O)_{10}}C{H_2}C{H_2}OH $
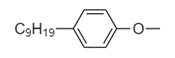
D. Hydrophobic part: $ - {(C{H_2}C{H_2}O)_{10}}C{H_2}C{H_2}OH $
Hydrophilic part:
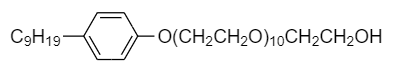




Answer
524.7k+ views
Hint :Detergent: It is a surfactant or combination of surfactants that shows cleansing properties when diluted in water. These are comparatively more soluble in hard water than soap because it contains sulfonate ions which do not interact with the magnesium and calcium ions present in the hard water.
Complete Step By Step Answer:
Detergent is amphiphilic in nature that means its structure consists of two regions which are hydrophilic region and hydrophobic region.
Hydrophilic region: The part of the detergent which interacts with the water molecule to form a bond is known as its hydrophilic part. In simple words, we can say that this region of detergent is water loving.
Hydrophobic region: The nonpolar part of the detergent which does not interact or repel the water molecules is termed as its hydrophobic part. In simple words, we can say that this region of detergent is water hating.
In the given non-ionic detergent, $ - O{(C{H_2}C{H_2}O)_{10}}C{H_2}C{H_2}OH $ is the polar part because of the presence of hydroxyl group. The $ OH $ group has a tendency to release a proton and hence is polar in nature. Therefore, it is the hydrophilic part of the detergent.
The remaining part of detergent is a hydrocarbon i.e., consisting of carbon and nitrogen atoms only. So it is non-polar. Hence hydrophobic part of detergent is as follows:
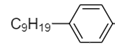
Thus, option (B) is the correct answer.
Note :
Non-ionic detergent: These are the types of detergents which form electrically neutral particles in the solution. These are neither cationic nor anionic in nature. These are less hard as compared to ionic detergents. The hydrophilic part of non-ionic detergents is uncharged.
Complete Step By Step Answer:
Detergent is amphiphilic in nature that means its structure consists of two regions which are hydrophilic region and hydrophobic region.
Hydrophilic region: The part of the detergent which interacts with the water molecule to form a bond is known as its hydrophilic part. In simple words, we can say that this region of detergent is water loving.
Hydrophobic region: The nonpolar part of the detergent which does not interact or repel the water molecules is termed as its hydrophobic part. In simple words, we can say that this region of detergent is water hating.
In the given non-ionic detergent, $ - O{(C{H_2}C{H_2}O)_{10}}C{H_2}C{H_2}OH $ is the polar part because of the presence of hydroxyl group. The $ OH $ group has a tendency to release a proton and hence is polar in nature. Therefore, it is the hydrophilic part of the detergent.
The remaining part of detergent is a hydrocarbon i.e., consisting of carbon and nitrogen atoms only. So it is non-polar. Hence hydrophobic part of detergent is as follows:

Thus, option (B) is the correct answer.
Note :
Non-ionic detergent: These are the types of detergents which form electrically neutral particles in the solution. These are neither cationic nor anionic in nature. These are less hard as compared to ionic detergents. The hydrophilic part of non-ionic detergents is uncharged.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE

Why was the Vernacular Press Act passed by British class 11 social science CBSE




