
Identify the functional group present in acetic acid:
A.
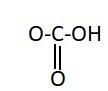
B.
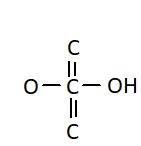
C.
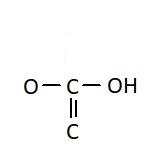
D.
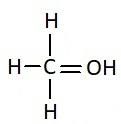




Answer
573.3k+ views
Hint: Before identifying the functional group in acetic acid, let’s find out what a functional group is. Functional groups are the atoms or groups or molecules which decide the reactivity of the molecules to which they are attached.
Complete step by step answer:
Sometimes, certain elements can also be considered as functional groups. Few examples are oxygen, nitrogen, phosphorus, sulfur etc. Alkanes are generally unreactive when compared to other functional groups. Thus they are not considered as functional groups. But alkenes are considered as functional groups. Alkenes are compounds which are represented as ${{C = C}}$. Similarly, each functional group has their own symbols like alcohols have ${{ - OH}}$ group, ethers have $ - {{O}}$ etc. Alcohols have a suffix “ol” at the end of the chemical name.
Similarly, the chemical names having “acid” as suffix are generally carboxylic acids. They have a carboxyl group attached to it. Carboxylic groups are involved in the carbonyl compounds. Carbonyl means they have a carbon atom which is double bonded to oxygen atom. Carboxylic groups have a carbonyl group, $ - {{C = O}}$ and a hydroxyl group, $ - {{OH}}$. Thus they have two oxygen atoms, i.e. one from carbonyl and one from hydroxyl group. The hydroxyl group and carbonyl group is attached to the same carbon. Moreover, the carboxylic group usually comes at the end of the carbon chain. They are also known as alkanoic acid. The term “oic acid” is used for naming the carboxylic acids. “Acetic” is generally used for compounds having two carbon atoms. Thus the chemical formula of acetic acid is ${{C}}{{{H}}_3}{{COOH}}$. Thus the functional group in acetic acid is represented as:
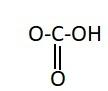
Hence, the correct option is A.
Note: The functional groups in each type of organic compounds are specific. They have a major role in formation of DNA, proteins, etc. Such functional groups involve hydroxyl $ - {{OH}}$, methyl $ - {{C}}{{{H}}_3}$, amino $ - {{N}}{{{H}}_2}$, carboxyl $ - {{COOH}}$ etc.
Complete step by step answer:
Sometimes, certain elements can also be considered as functional groups. Few examples are oxygen, nitrogen, phosphorus, sulfur etc. Alkanes are generally unreactive when compared to other functional groups. Thus they are not considered as functional groups. But alkenes are considered as functional groups. Alkenes are compounds which are represented as ${{C = C}}$. Similarly, each functional group has their own symbols like alcohols have ${{ - OH}}$ group, ethers have $ - {{O}}$ etc. Alcohols have a suffix “ol” at the end of the chemical name.
Similarly, the chemical names having “acid” as suffix are generally carboxylic acids. They have a carboxyl group attached to it. Carboxylic groups are involved in the carbonyl compounds. Carbonyl means they have a carbon atom which is double bonded to oxygen atom. Carboxylic groups have a carbonyl group, $ - {{C = O}}$ and a hydroxyl group, $ - {{OH}}$. Thus they have two oxygen atoms, i.e. one from carbonyl and one from hydroxyl group. The hydroxyl group and carbonyl group is attached to the same carbon. Moreover, the carboxylic group usually comes at the end of the carbon chain. They are also known as alkanoic acid. The term “oic acid” is used for naming the carboxylic acids. “Acetic” is generally used for compounds having two carbon atoms. Thus the chemical formula of acetic acid is ${{C}}{{{H}}_3}{{COOH}}$. Thus the functional group in acetic acid is represented as:

Hence, the correct option is A.
Note: The functional groups in each type of organic compounds are specific. They have a major role in formation of DNA, proteins, etc. Such functional groups involve hydroxyl $ - {{OH}}$, methyl $ - {{C}}{{{H}}_3}$, amino $ - {{N}}{{{H}}_2}$, carboxyl $ - {{COOH}}$ etc.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




