
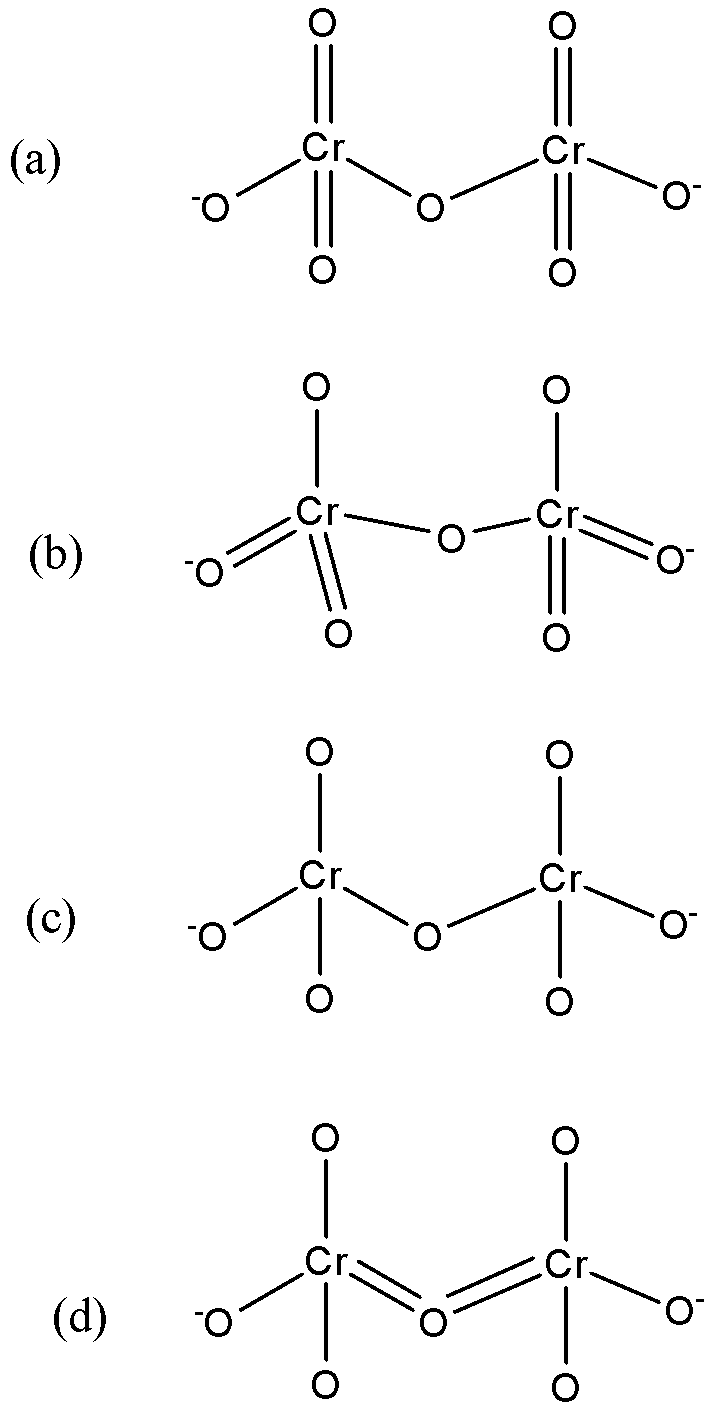
Identify the correct structure of dichromate ion:

Answer
602.4k+ views
Hint: The correct structure has 4 double bonds. All the 4 double bonds are arranged in such a manner that they have the least repulsion and they have symmetry in the structure.
Complete step by step solution:
The formula of dichromate is $C{{r}_{2}}{{O}_{7}}^{2-}$.
The dichromate structure has 2 chromium ions as central metal ions forming a bridge with a common oxygen atom.
It has four double bonds- 2 on each chromium ions in such a manner that they are in the opposite direction.
The structure is:
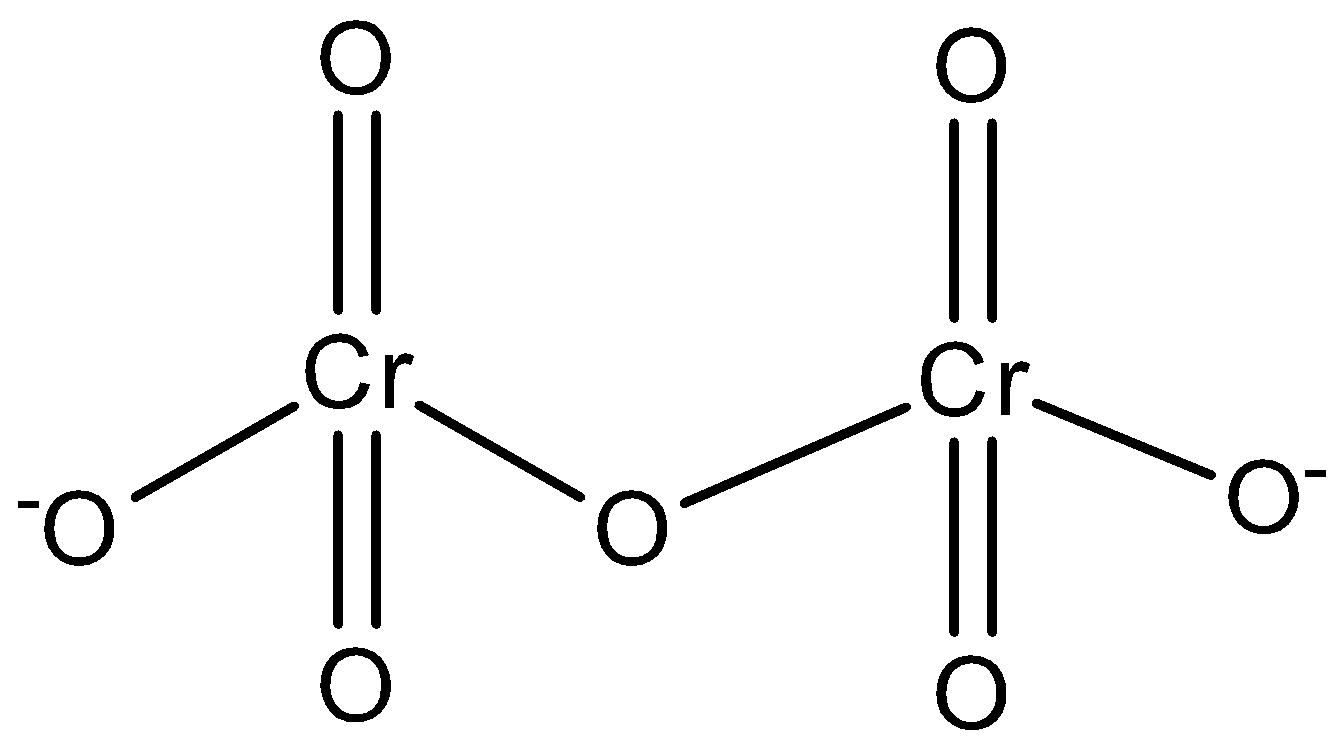
In $C{{r}_{2}}{{O}_{7}}^{2-}$ ion, the two Cr-O bonds which share an oxygen atom at the common vertex of two tetrahedral units are longer than the other six equivalent bonds.
The bond length of Cr and the common O atom is 179pm.
All the other Cr-O bonds are 163pm.
The bond angle of the oxygen atom which is bridged between 2 chromium ions has ${{126}^{\circ }}$.
It is important to know that in both $Cr{{O}_{4}}^{2-}$ and $C{{r}_{2}}{{O}_{7}}^{2-}$ and ions, Cr (VI) has ${{d}^{0}}$a configuration. Hence, the yellow color of $Cr{{O}_{4}}^{2-}$ the orange color $C{{r}_{2}}{{O}_{7}}^{2-}$ is not due to d-d transition but due to charge transfer, i.e., momentary transfer of charge from O-atom to metal atom thereby changing ${{O}^{2-}}$ ion momentarily to ${{O}^{-}}$ ion and reducing the oxidation state of chromium atom from +6 to +5.
So, the correct answer is “Option A”.
Note: You might get confused between the option (a) and (c) because the structure of (c) is also referred but the difference is that in option (c) the 2- charge is on the whole molecule. But the actual structure is an option (a).
Complete step by step solution:
The formula of dichromate is $C{{r}_{2}}{{O}_{7}}^{2-}$.
The dichromate structure has 2 chromium ions as central metal ions forming a bridge with a common oxygen atom.
It has four double bonds- 2 on each chromium ions in such a manner that they are in the opposite direction.
The structure is:

In $C{{r}_{2}}{{O}_{7}}^{2-}$ ion, the two Cr-O bonds which share an oxygen atom at the common vertex of two tetrahedral units are longer than the other six equivalent bonds.
The bond length of Cr and the common O atom is 179pm.
All the other Cr-O bonds are 163pm.
The bond angle of the oxygen atom which is bridged between 2 chromium ions has ${{126}^{\circ }}$.
It is important to know that in both $Cr{{O}_{4}}^{2-}$ and $C{{r}_{2}}{{O}_{7}}^{2-}$ and ions, Cr (VI) has ${{d}^{0}}$a configuration. Hence, the yellow color of $Cr{{O}_{4}}^{2-}$ the orange color $C{{r}_{2}}{{O}_{7}}^{2-}$ is not due to d-d transition but due to charge transfer, i.e., momentary transfer of charge from O-atom to metal atom thereby changing ${{O}^{2-}}$ ion momentarily to ${{O}^{-}}$ ion and reducing the oxidation state of chromium atom from +6 to +5.
So, the correct answer is “Option A”.
Note: You might get confused between the option (a) and (c) because the structure of (c) is also referred but the difference is that in option (c) the 2- charge is on the whole molecule. But the actual structure is an option (a).
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




