
Identify the correct Lewis symbol from the following:
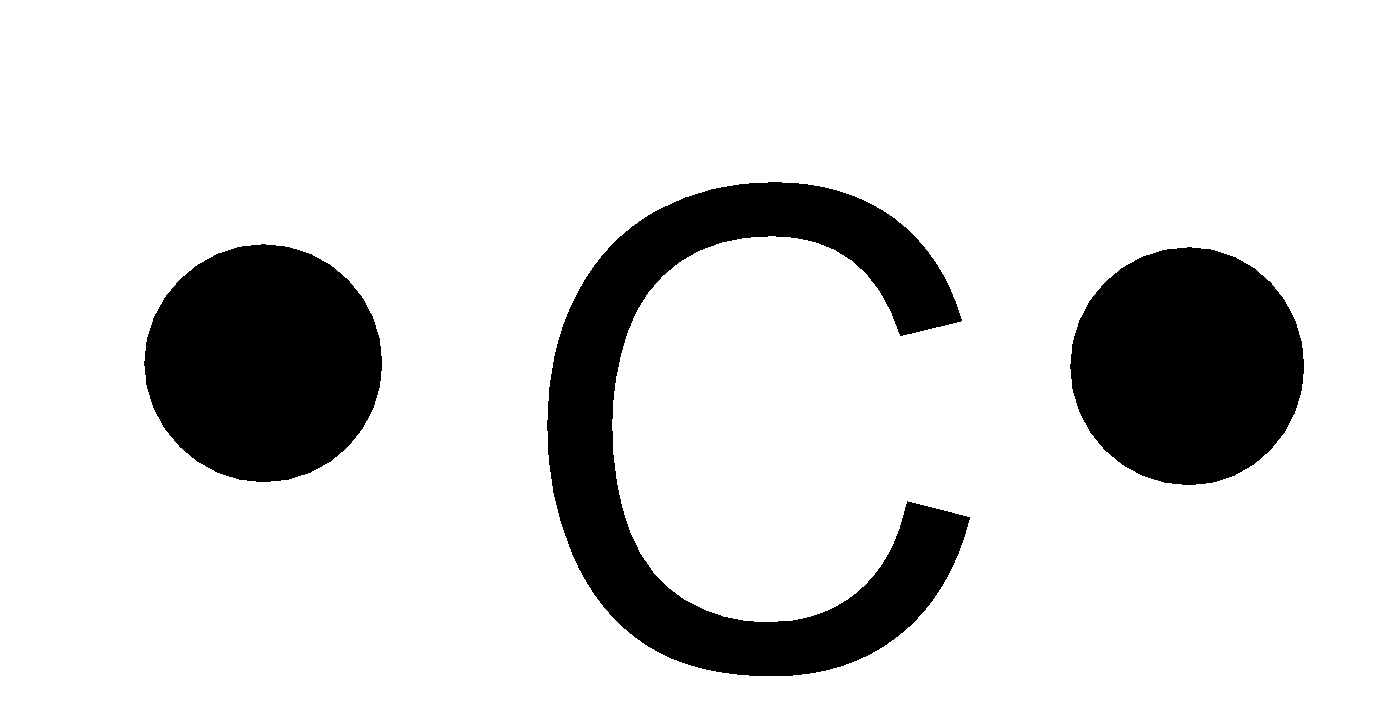
A.
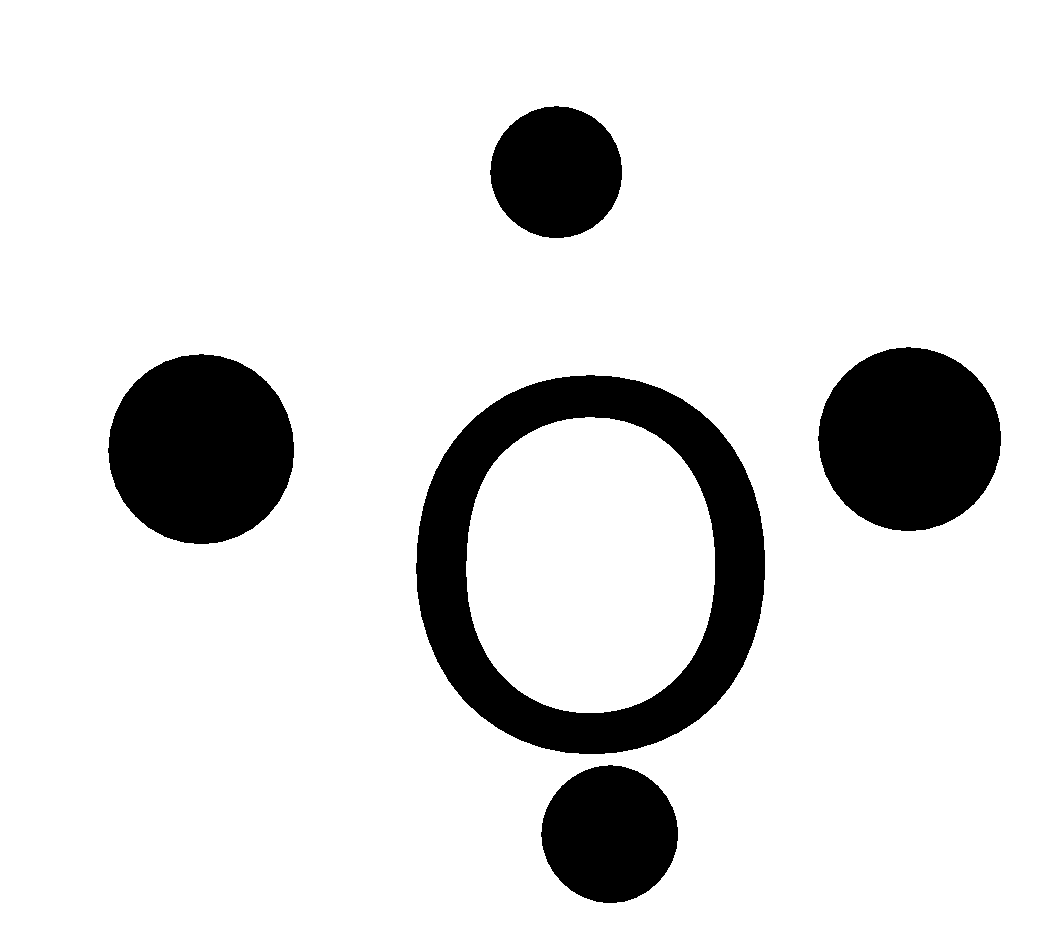
B.
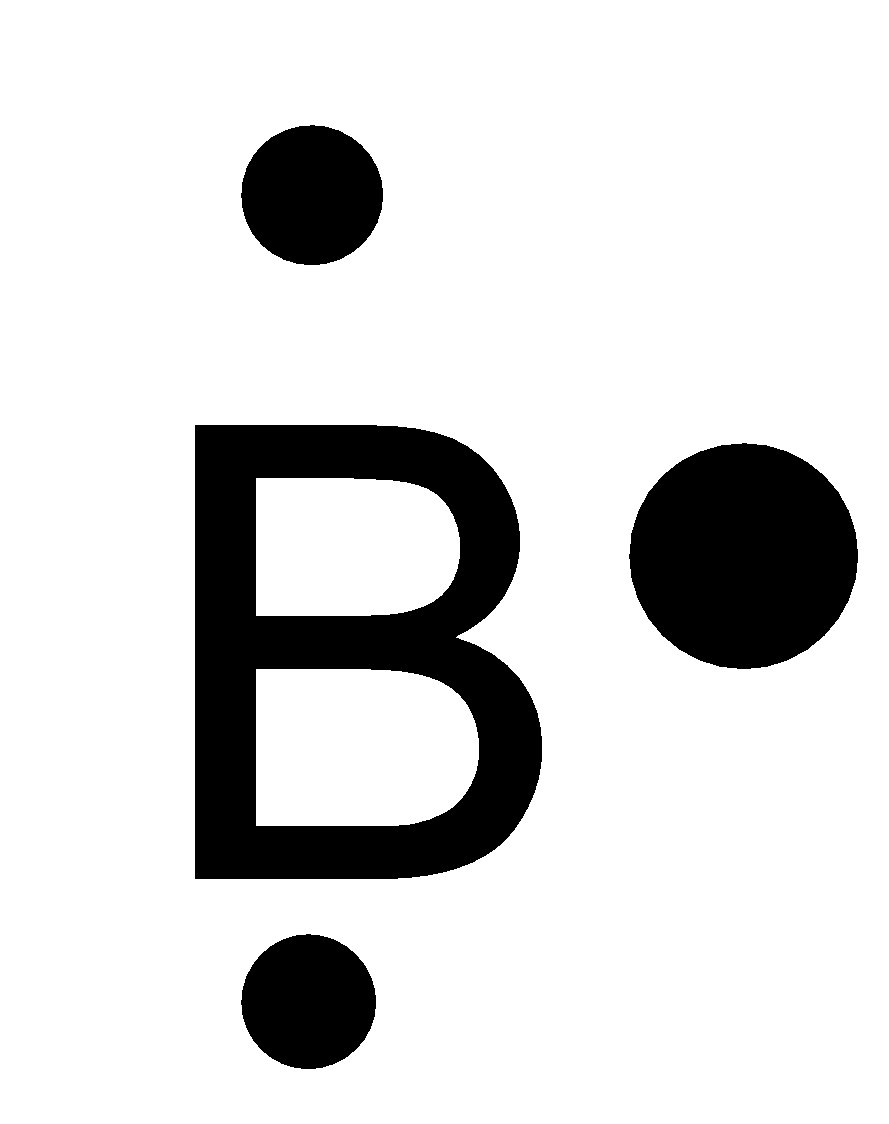
C.

D.
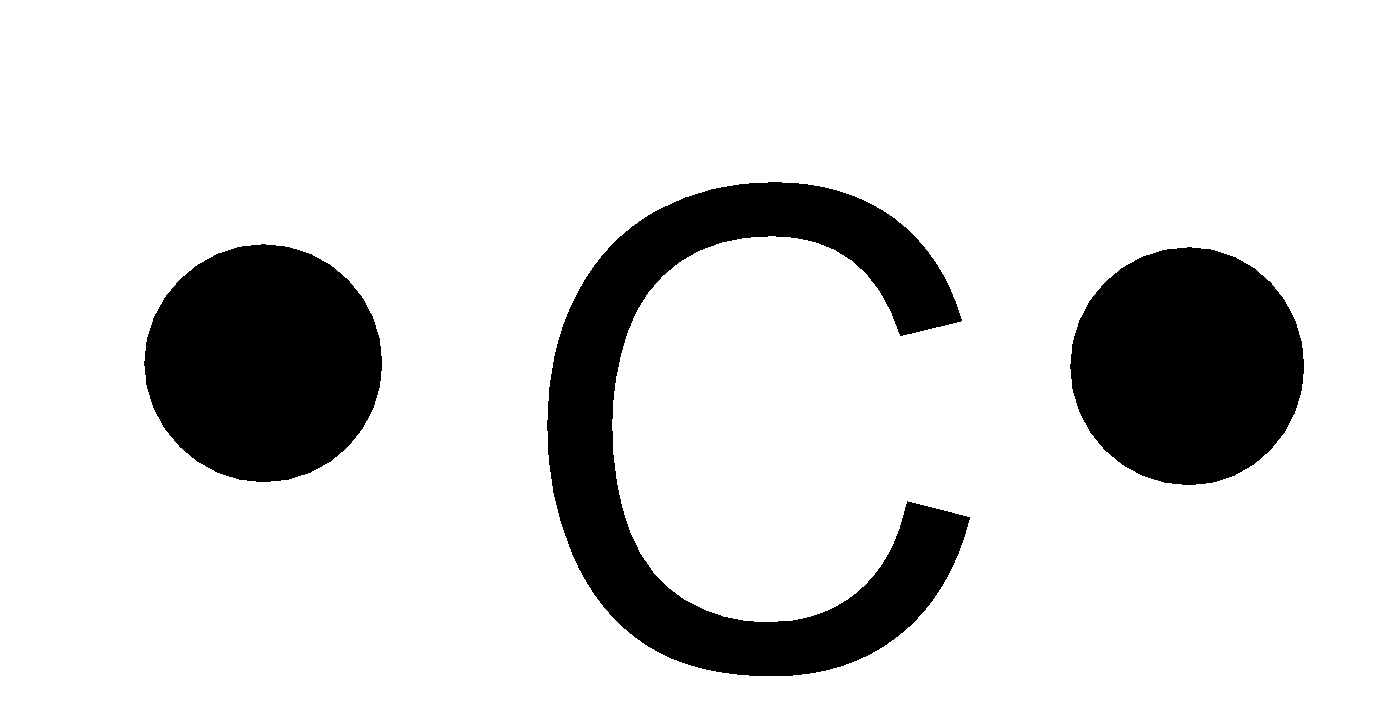



Answer
594k+ views
Hint: Lewis structures or Lewis electron dot structures or Lewis symbols are simplified diagrams or representations of the valence shell electrons in a molecule. These structures are used to display how the electrons are arranged around the individual atoms in a molecule. The electrons are represented as dots and for bonding electrons as a line between the two atoms.
Complete step by step answer:
-A Lewis electron dot structure can be drawn for a single atom, a polyatomic ion or a covalent compound. In Lewis structures, only valence electrons are represented. Non-valence electrons are not represented. The ultimate aim of drawing electron dot structures is to fulfill the octet rule.
-The periodic table is a very good reference for drawing Lewis electron dot structures. The Roman numeral which is the numbering of a group represents the number of valence electrons of the elements of that group. For example, the elements of group I will have one valence electron, the elements of group II will have two valence electrons and so on.
-The Lewis dot structure is denoted by an atomic symbol with the dots representing the valence electrons arranged around it.
-Carbon is an element of group 14 and has the atomic number 6 and atomic symbol C. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 4 valence electrons. So, the Lewis symbol for carbon is:

So, option A is not correct.
-Oxygen is a group 16 element, has the atomic number 8 and atomic symbol O. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 6 valence electrons. So, the Lewis symbol for oxygen is:
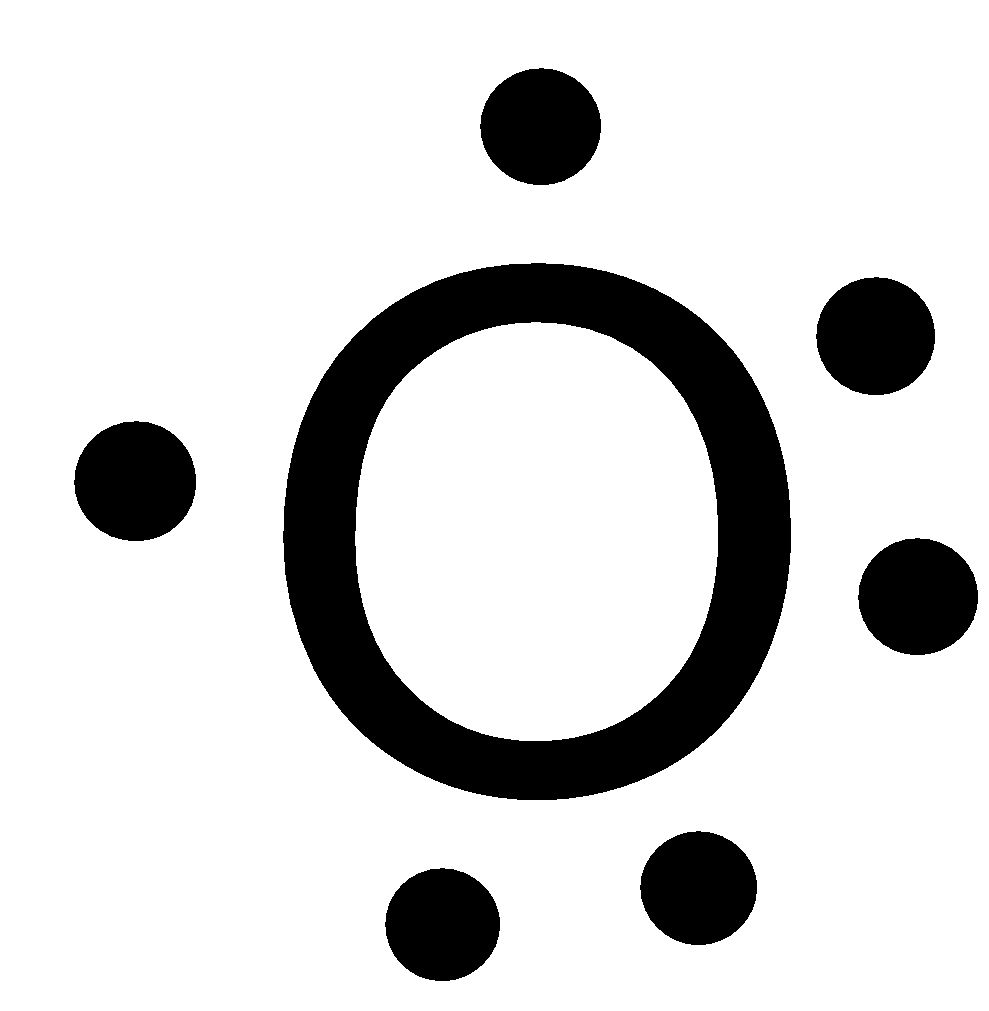
So, option B is not correct.
-Boron is a group 13 element, has the atomic number 5 and atomic symbol B. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 3 valence electrons. So, the Lewis symbol for boron is:
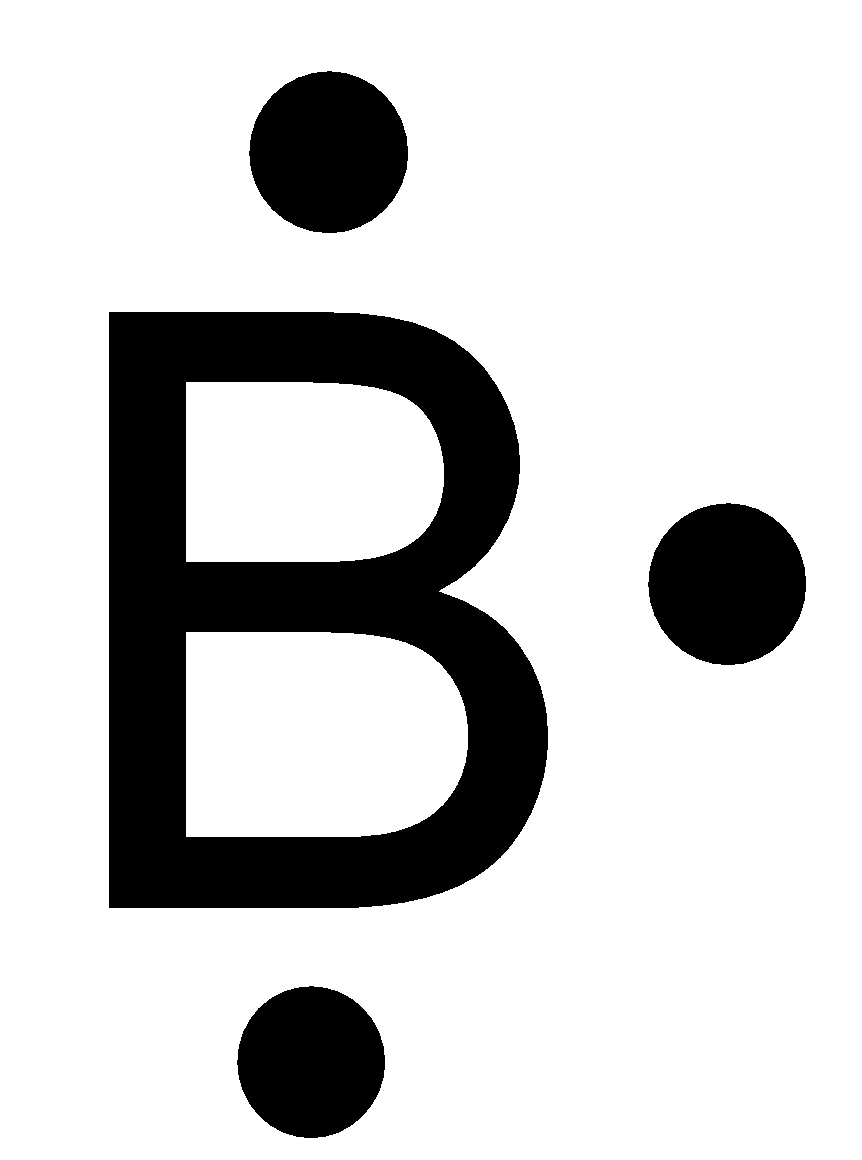
So, option C is correct.
-Nitrogen is a group 15 element, has the atomic number 7 and atomic symbol N. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 5 valence electrons. So, the Lewis symbol for nitrogen is:

So, option D is not correct.
Hence option C is correct.
Note: Lewis electron dot structure is the simplest and also the most limited theory or concept on electronic structure. The Lewis electron dot structure does not attempt to interpret the geometry of the atoms or molecules or how the bonds are formed between two atoms or how the electrons are shared between atoms.
Complete step by step answer:
-A Lewis electron dot structure can be drawn for a single atom, a polyatomic ion or a covalent compound. In Lewis structures, only valence electrons are represented. Non-valence electrons are not represented. The ultimate aim of drawing electron dot structures is to fulfill the octet rule.
-The periodic table is a very good reference for drawing Lewis electron dot structures. The Roman numeral which is the numbering of a group represents the number of valence electrons of the elements of that group. For example, the elements of group I will have one valence electron, the elements of group II will have two valence electrons and so on.
-The Lewis dot structure is denoted by an atomic symbol with the dots representing the valence electrons arranged around it.
-Carbon is an element of group 14 and has the atomic number 6 and atomic symbol C. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 4 valence electrons. So, the Lewis symbol for carbon is:

So, option A is not correct.
-Oxygen is a group 16 element, has the atomic number 8 and atomic symbol O. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 6 valence electrons. So, the Lewis symbol for oxygen is:

So, option B is not correct.
-Boron is a group 13 element, has the atomic number 5 and atomic symbol B. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 3 valence electrons. So, the Lewis symbol for boron is:

So, option C is correct.
-Nitrogen is a group 15 element, has the atomic number 7 and atomic symbol N. It lies in the second period. So, it can fit a maximum of 8 electrons in the second energy level and there are 5 valence electrons. So, the Lewis symbol for nitrogen is:

So, option D is not correct.
Hence option C is correct.
Note: Lewis electron dot structure is the simplest and also the most limited theory or concept on electronic structure. The Lewis electron dot structure does not attempt to interpret the geometry of the atoms or molecules or how the bonds are formed between two atoms or how the electrons are shared between atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




