
Identify the combination of compounds that undergo aldol condensation followed by dehydration to produce but 2 enal.
A. Methanal and ethanal
B. Two moles of ethanal
C. Methanal and propanone
D. Ethanal and propanone
Answer
579.6k+ views
Hint: We can identify the combination of compounds by doing the reverse of an aldol condensation. The reactants of aldol condensation are aldehyde molecules. To get the aldehyde molecules, add water on the double bond to gain beta-hydroxy aldehyde. Then break the alpha bond of aldehyde gets aldehydes that are reacting.
Complete step by step solution:
In aldol condensation, two aldehyde molecules react in presence of a base to give beta-hydroxy aldehyde.
In the first step, base abstract alpha-proton forms an aldehyde. The carbanion undergoes keto-enol tautomerism.
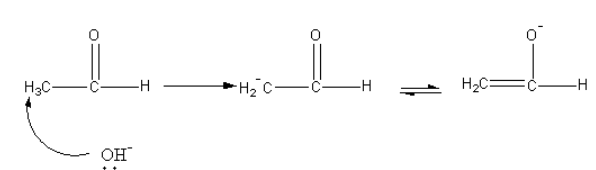
The nucleophilic carbon attack on the carbonyl carbon of another aldehyde.
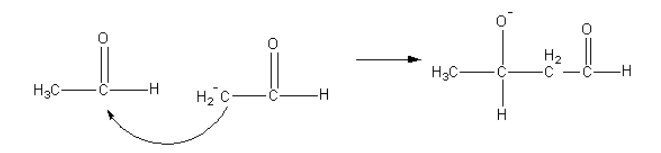
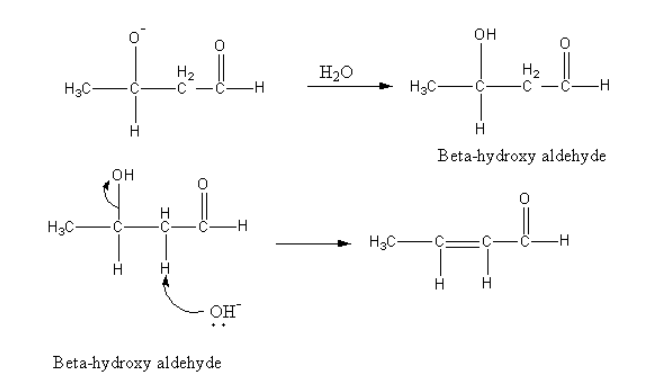
Then in presence of water negatively charged oxygen gets protonated and product beta-hydroxy aldehyde forms.
Further base abstract the alpha proton from beta-hydroxy aldehyde and the removal of the hydroxyl group give alpha, beta-unsaturated aldehyde.
The reverse of the aldol condensation can be done to determine the reactants, so we add a water molecule alpha-beta double bond which will give beta-hydroxy-aldehyde. On further dissociation two ethan-al molecules are obtained.
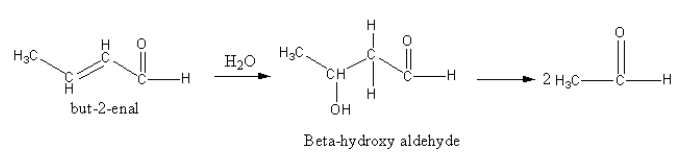
So, the combination of compounds that undergo aldol condensation followed by dehydration to produce but-2-enal is two moles of ethanal.
Therefore, option (B) Two moles of ethanal, is correct.
Note: The alpha-hydrogen having aldehydes gives an aldol condensation reaction. The aldehyde having alpha-hydrogen can undergo keto-enol tautomerism. The double-bonded carbon in keto form is more electrophilic than the carbon bonded with the hydroxyl group in enol form. The aldehyde molecules that cannot undergo keto-enol tautomerism give the Cannizzaro reaction. Cannizzaro reaction also takes place in the alkali solution.
Complete step by step solution:
In aldol condensation, two aldehyde molecules react in presence of a base to give beta-hydroxy aldehyde.
In the first step, base abstract alpha-proton forms an aldehyde. The carbanion undergoes keto-enol tautomerism.

The nucleophilic carbon attack on the carbonyl carbon of another aldehyde.

Then in presence of water negatively charged oxygen gets protonated and product beta-hydroxy aldehyde forms.
Further base abstract the alpha proton from beta-hydroxy aldehyde and the removal of the hydroxyl group give alpha, beta-unsaturated aldehyde.

The reverse of the aldol condensation can be done to determine the reactants, so we add a water molecule alpha-beta double bond which will give beta-hydroxy-aldehyde. On further dissociation two ethan-al molecules are obtained.

So, the combination of compounds that undergo aldol condensation followed by dehydration to produce but-2-enal is two moles of ethanal.
Therefore, option (B) Two moles of ethanal, is correct.
Note: The alpha-hydrogen having aldehydes gives an aldol condensation reaction. The aldehyde having alpha-hydrogen can undergo keto-enol tautomerism. The double-bonded carbon in keto form is more electrophilic than the carbon bonded with the hydroxyl group in enol form. The aldehyde molecules that cannot undergo keto-enol tautomerism give the Cannizzaro reaction. Cannizzaro reaction also takes place in the alkali solution.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




