
Identify if chloroform is insoluble, partially soluble or highly soluble in water?
Answer
574.8k+ views
Hint: Chloroform, also known as trichloromethane, is a volatile, colourless liquid possessing a distinct odour. It is a non-flammable material having the chemical formula of \[CHC{l_3}\].
Complete step by step solution: Solubility actually indicates the maximum amount of a substance which can be dissolved in solvent at a specific temperature. Generally, substances are soluble with each other if all the intermolecular forces of attraction are roughly the same. The main idea to remember is that like dissolves like. This means that polar solutes get dissolved in polar solvents and non-polar solutes get dissolved in non-polar solvents. Now, let us discuss the case of chloroform.
\[CHC{l_3}\] is slightly polar and water (\[{H_2}O\]) is polar. According to this, one should think that \[CHC{l_3}\] is soluble in \[{H_2}O\] but this is just a general way to forecast the solubility. One of the major factors to be considered is the distribution of molecules. Bipolar-bipolar bonding in \[CHC{l_3}\] as well as hydrogen bonding in \[{H_2}O\] is stronger in comparison to bipolar-bipolar bonding that exist between \[{H_2}O\] and \[CHC{l_3}\].
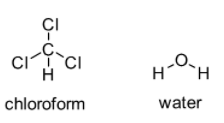
That means attraction between \[CHC{l_3}\] molecules is more compared to the attraction between \[CHC{l_3}\] and \[{H_2}O\]. Moreover attraction between \[{H_2}O\] molecules is also greater than the attraction between \[CHC{l_3}\] and \[{H_2}O\]. As a result, even being slightly polar \[CHC{l_3}\] is not soluble in \[{H_2}O\].
Hence, chloroform is insoluble in water.
Note: Alternatively, insolubility of chloroform in water can be explained on the basis of the inability of chloroform to make hydrogen bond in water. Hydrogen bonding occurs in case of nitrogen, oxygen, fluorine owing to the greater difference in the electronegativity between Hydrogen and nitrogen, oxygen, fluorine. But only a slight difference is there between the electronegativity of Hydrogen and Chlorine thus, no H-bonding can exist. Hence, chloroform is insoluble in water.
Complete step by step solution: Solubility actually indicates the maximum amount of a substance which can be dissolved in solvent at a specific temperature. Generally, substances are soluble with each other if all the intermolecular forces of attraction are roughly the same. The main idea to remember is that like dissolves like. This means that polar solutes get dissolved in polar solvents and non-polar solutes get dissolved in non-polar solvents. Now, let us discuss the case of chloroform.
\[CHC{l_3}\] is slightly polar and water (\[{H_2}O\]) is polar. According to this, one should think that \[CHC{l_3}\] is soluble in \[{H_2}O\] but this is just a general way to forecast the solubility. One of the major factors to be considered is the distribution of molecules. Bipolar-bipolar bonding in \[CHC{l_3}\] as well as hydrogen bonding in \[{H_2}O\] is stronger in comparison to bipolar-bipolar bonding that exist between \[{H_2}O\] and \[CHC{l_3}\].

That means attraction between \[CHC{l_3}\] molecules is more compared to the attraction between \[CHC{l_3}\] and \[{H_2}O\]. Moreover attraction between \[{H_2}O\] molecules is also greater than the attraction between \[CHC{l_3}\] and \[{H_2}O\]. As a result, even being slightly polar \[CHC{l_3}\] is not soluble in \[{H_2}O\].
Hence, chloroform is insoluble in water.
Note: Alternatively, insolubility of chloroform in water can be explained on the basis of the inability of chloroform to make hydrogen bond in water. Hydrogen bonding occurs in case of nitrogen, oxygen, fluorine owing to the greater difference in the electronegativity between Hydrogen and nitrogen, oxygen, fluorine. But only a slight difference is there between the electronegativity of Hydrogen and Chlorine thus, no H-bonding can exist. Hence, chloroform is insoluble in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




