
Identify A
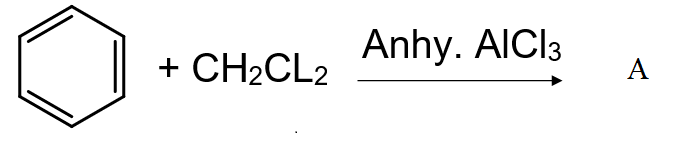
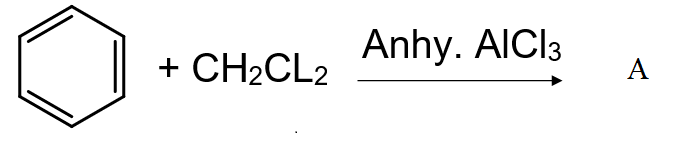
Answer
586.2k+ views
Hint: To answer this question, you should recall the concept of electrophilic substitution reaction and Friedel craft alkylation. An electrophilic substitution reaction is a chemical reaction in which the functional group attached to a compound is replaced by an electrophile.
Complete step by step answer:
Reactions involving electrophilic substitution reactions generally proceed via a three-step mechanism that involve: The generation of an electrophile, the formation of a carbocation and the removal of a proton from the intermediate. Friedel-Crafts Alkylation refers to the replacement of an aromatic proton with an alkyl group. This is done through an electrophilic attack on the aromatic ring with the help of a carbocation. The Friedel-Crafts alkylation reaction is a method of generating alkylbenzenes by using alkyl halides as reactants.
A Lewis acid catalyst such as \[FeC{l_3}\;{\text{or }}AlC{l_3}\;\] vis employed in this reaction in order to form a carbocation by facilitating the removal of the halide. The resulting carbocation undergoes a rearrangement before proceeding with the alkylation reaction.
Step 1: The catalyst which is \[AlC{l_3}\] undergoes reaction with the alkyl halide, resulting in the formation of an electrophilic carbocation.
Step 2: The carbocation proceeds to attack the aromatic ring, forming a cyclohexadienyl cation as an intermediate. The aromaticity of the arene is temporarily lost due to the breakage of the carbon-carbon double bond.
Step 3: The deprotonation of the intermediate leads to the reformation of the carbon-carbon double bond, restoring aromaticity to the compound. This proton goes on to form hydrochloric acid, regenerating the \[AlC{l_3}\] catalyst.
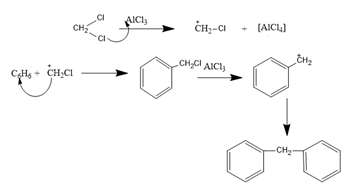
The process can be represented by the following equation:
Note:
You should know about the limitations of the Friedel-Crafts Alkylation Reaction: Some important limitations of Friedel-Crafts alkylation are:
Since the carbocations formed by aryl and vinyl halides are extremely unstable, they cannot be used in this reaction. The presence of a deactivating group on the aromatic ring can lead to the deactivation of the catalyst due to the formation of complexes. An excess of the aromatic compound must be used in these reactions in order to avoid polyalkylation.
Aromatic compounds that are less reactive than mono-halobenzenes do not participate in the Friedel-Crafts alkylation reaction. It is important to note that this reaction is prone to carbocation rearrangements, as is the case with any reaction involving carbocations.
Complete step by step answer:
Reactions involving electrophilic substitution reactions generally proceed via a three-step mechanism that involve: The generation of an electrophile, the formation of a carbocation and the removal of a proton from the intermediate. Friedel-Crafts Alkylation refers to the replacement of an aromatic proton with an alkyl group. This is done through an electrophilic attack on the aromatic ring with the help of a carbocation. The Friedel-Crafts alkylation reaction is a method of generating alkylbenzenes by using alkyl halides as reactants.
A Lewis acid catalyst such as \[FeC{l_3}\;{\text{or }}AlC{l_3}\;\] vis employed in this reaction in order to form a carbocation by facilitating the removal of the halide. The resulting carbocation undergoes a rearrangement before proceeding with the alkylation reaction.
Step 1: The catalyst which is \[AlC{l_3}\] undergoes reaction with the alkyl halide, resulting in the formation of an electrophilic carbocation.
Step 2: The carbocation proceeds to attack the aromatic ring, forming a cyclohexadienyl cation as an intermediate. The aromaticity of the arene is temporarily lost due to the breakage of the carbon-carbon double bond.
Step 3: The deprotonation of the intermediate leads to the reformation of the carbon-carbon double bond, restoring aromaticity to the compound. This proton goes on to form hydrochloric acid, regenerating the \[AlC{l_3}\] catalyst.
The process can be represented by the following equation:

Note:
You should know about the limitations of the Friedel-Crafts Alkylation Reaction: Some important limitations of Friedel-Crafts alkylation are:
Since the carbocations formed by aryl and vinyl halides are extremely unstable, they cannot be used in this reaction. The presence of a deactivating group on the aromatic ring can lead to the deactivation of the catalyst due to the formation of complexes. An excess of the aromatic compound must be used in these reactions in order to avoid polyalkylation.
Aromatic compounds that are less reactive than mono-halobenzenes do not participate in the Friedel-Crafts alkylation reaction. It is important to note that this reaction is prone to carbocation rearrangements, as is the case with any reaction involving carbocations.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




