
Hydrazobenzene can be obtained from nitro benzene in the presence of:
(A) \[Zn + KOH\]
(B) \[Zn + HCl\]
(C) \[Zn + ZnO\]
(D) \[Zn + {H_2}S{O_4}\]
Answer
570k+ views
Hint: For solving this problem we should know the reactions of nitrobenzene. Nitrobenzene is acidic in nature so we have to study the properties of them as well. And we need to know all the possible ways to prepare hydrazobenzene. Then only we can be able to solve the above problem. And also consider the given reagents and their reaction with nitrobenzene to answer the question correctly.
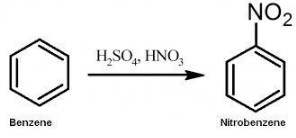
Complete step-by-step answer:Nitrobenzene is an organic compound having the chemical formula \[{C_6}{H_5}N{O_2}\]. It is a water insoluble compound with almond like odour and a pale yellow outlook. Nitrobenzene is obtained from nitration of benzene. Nitration reaction occurs in presence of benzene and mixed acid (\[{H_2}S{O_4}\],\[{H_2}O\]and\[HN{O_3}\]).
Hydrazobenzene is a derivative of hydrazine. It is a colorless crystal at room temperature. It is practically insoluble in water, slightly soluble in benzene, completely soluble in ethanol.
It is also called \[1,2 - diphenylhydrazine\]. It is an important chemical for industrial uses and also used widely in preparation of hydrogen peroxide.
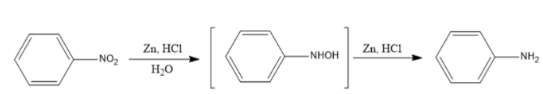
If we talk about the reaction of nitrobenzene with \[Zn + HCl\]:
Nitrobenzene will be reduced in presence of \[Zn + HCl\] and aniline will be produced.
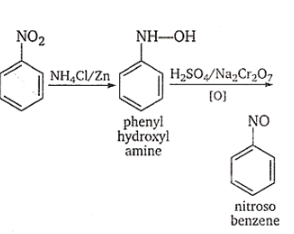
If nitrobenzene is treated with \[Zn\]dust and \[{H_2}S{O_4}\]it will produce nitrosobenzene.
And finally, if we treat nitrobenzene with \[Zn\]dust and alcohol \[KOH\]we will get hydrazobenzene as a product.
\[{C_6}{H_5}N{O_2} + Zn\;Powder + alcoholicKOH \to {C_6}{H_5} - NH - NH - {C_6}{H_5}\]
So, the answer will be option (A)
Note:considering all the reactions with nitrobenzene we will get the answer. \[Zn\]Dust and \[KOH\]and also sodium amalgam and carbon will be required for the completion of the reaction. In sodium amalgam it is necessary to have \[0.1 - 0.2\% \]sodium.
Complete step-by-step answer:Nitrobenzene is an organic compound having the chemical formula \[{C_6}{H_5}N{O_2}\]. It is a water insoluble compound with almond like odour and a pale yellow outlook. Nitrobenzene is obtained from nitration of benzene. Nitration reaction occurs in presence of benzene and mixed acid (\[{H_2}S{O_4}\],\[{H_2}O\]and\[HN{O_3}\]).

Hydrazobenzene is a derivative of hydrazine. It is a colorless crystal at room temperature. It is practically insoluble in water, slightly soluble in benzene, completely soluble in ethanol.
It is also called \[1,2 - diphenylhydrazine\]. It is an important chemical for industrial uses and also used widely in preparation of hydrogen peroxide.
If we talk about the reaction of nitrobenzene with \[Zn + HCl\]:
Nitrobenzene will be reduced in presence of \[Zn + HCl\] and aniline will be produced.

If nitrobenzene is treated with \[Zn\]dust and \[{H_2}S{O_4}\]it will produce nitrosobenzene.

And finally, if we treat nitrobenzene with \[Zn\]dust and alcohol \[KOH\]we will get hydrazobenzene as a product.
\[{C_6}{H_5}N{O_2} + Zn\;Powder + alcoholicKOH \to {C_6}{H_5} - NH - NH - {C_6}{H_5}\]
So, the answer will be option (A)
Note:considering all the reactions with nitrobenzene we will get the answer. \[Zn\]Dust and \[KOH\]and also sodium amalgam and carbon will be required for the completion of the reaction. In sodium amalgam it is necessary to have \[0.1 - 0.2\% \]sodium.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




