
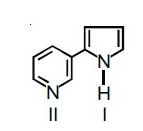
Hybridization of Nitrogen I and II in the following compound:
A. $s{{p}^{2}},s{{p}^{3}}$
B. $sp,s{{p}^{2}}$
C. $s{{p}^{2}},s{{p}^{2}}$
D. $s{{p}^{2}},sp$

Answer
573k+ views
Hint: Hybridization can be defined as the mixing of two atomic orbitals which have the same energy levels and by this mixing they provide a degenerated new type of orbitals and the mixing is based on quantum mechanics.
Complete Solution :
Hybridization can also be defined as the redistribution of the energy of orbitals of individual atoms to give orbitals of equivalent energy happens when two atomic orbitals combine to form hybrid orbital in a molecule and the new orbitals formed in this process are known as hybrid orbitals. On the basis of mixing hybridization can be classified in 6 categories which can be shown as: \[sp,s{{p}^{2}},s{{p}^{3}},s{{p}^{3}}d,s{{p}^{3}}{{d}^{2}},s{{p}^{3}}{{d}^{3}}\].
- In the given question nitrogen atom present at I position in this case nitrogen lone pairs takes part in the resonance to make double bond and due to this the compound is aromatic in nature so we can say there are only 3 bond pairs due to which it acquire \[s{{p}^{2}}\] hybridization while in II compound lone pair present on the nitrogen atom does not participate in the resonance process so it also shows \[s{{p}^{2}}\] hybridization.
So we can say that hybridization of Nitrogen I and II in the given compound is \[s{{p}^{2}}\].
So, the correct answer is “Option C”.
Note: Atomic orbitals which have equal energy can undergo the process of hybridization and the number of hybrid orbitals are equal to the number of atomic orbitals mixed. The main application of hybridization is we can predict the shape of molecules.
Complete Solution :
Hybridization can also be defined as the redistribution of the energy of orbitals of individual atoms to give orbitals of equivalent energy happens when two atomic orbitals combine to form hybrid orbital in a molecule and the new orbitals formed in this process are known as hybrid orbitals. On the basis of mixing hybridization can be classified in 6 categories which can be shown as: \[sp,s{{p}^{2}},s{{p}^{3}},s{{p}^{3}}d,s{{p}^{3}}{{d}^{2}},s{{p}^{3}}{{d}^{3}}\].
- In the given question nitrogen atom present at I position in this case nitrogen lone pairs takes part in the resonance to make double bond and due to this the compound is aromatic in nature so we can say there are only 3 bond pairs due to which it acquire \[s{{p}^{2}}\] hybridization while in II compound lone pair present on the nitrogen atom does not participate in the resonance process so it also shows \[s{{p}^{2}}\] hybridization.
So we can say that hybridization of Nitrogen I and II in the given compound is \[s{{p}^{2}}\].
So, the correct answer is “Option C”.
Note: Atomic orbitals which have equal energy can undergo the process of hybridization and the number of hybrid orbitals are equal to the number of atomic orbitals mixed. The main application of hybridization is we can predict the shape of molecules.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




