
What is the hybridisation of boron and nitrogen in borazine?
A) Both $s{p^3}$
B) N is $s{p^3}$ and B is $s{p^2}$ hybridised
C) Both $s{p^2}$
D) N is $s{p^2}$ and B is $s{p^2}$ hybridised
Answer
573.9k+ views
Hint: Borazine is ${B_3}{N_3}{H_6}$. It has very similarities with the benzene molecule and thus, it is also aromatic. There is a sideways overlap between the filled p-orbitals of N atom and empty p-orbitals of B atom within the borazine ring.
Complete Solution :
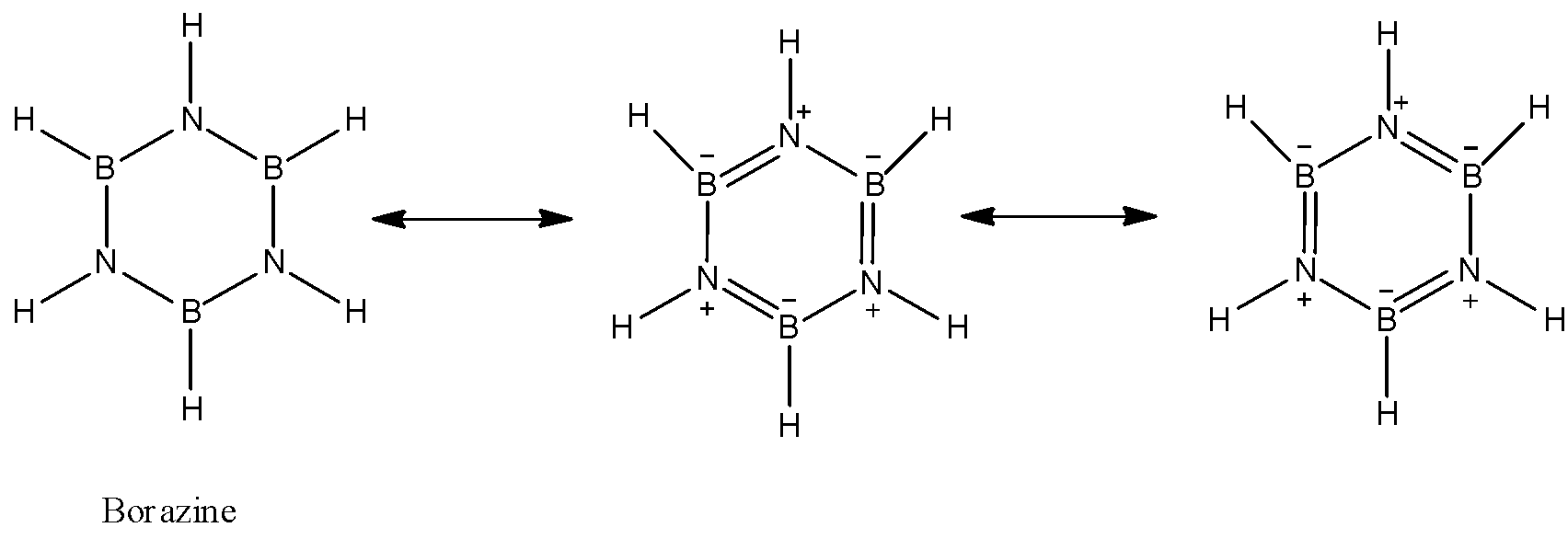
- Chemical formula of Borazine is ${B_3}{N_3}{H_6}$. Various chemical reactions and electron diffraction studies show that borazine is isoelectronic with the organic molecule i.e., Benzene and hence its structure is same as that of benzene. Like benzene, borazine also has a planar hexagonal structure containing 6 membered rings, in which Boron (B) and Nitrogen (N) atoms are arranged alternately. Because of the similarities between the structures of borazine and benzene, borazine is called as inorganic benzene.
Structure of borazine:
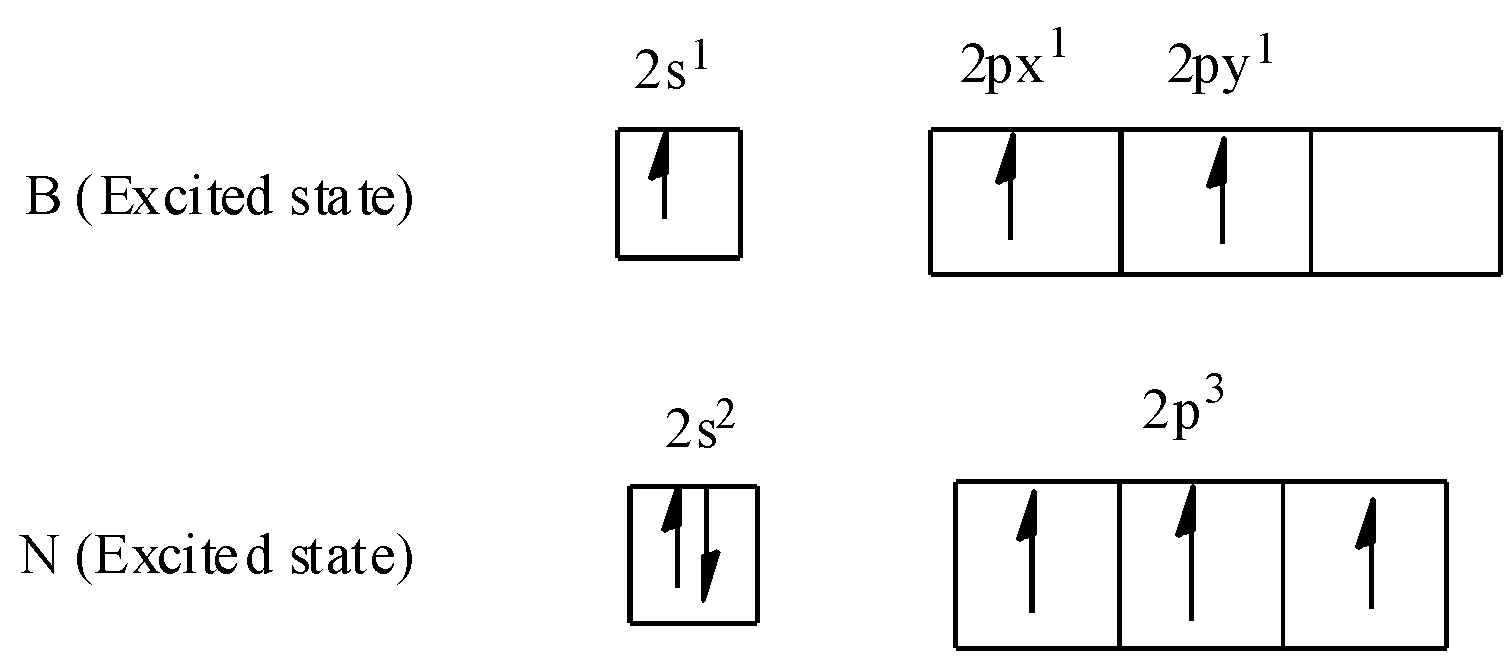
Electronic configuration of Boron, B in ground state: $1{s^2}2{s^2}2{p^1}$.
Electronic configuration of Nitrogen, N in ground state: $1{s^2}2{s^2}2{p^3}$.
Each nitrogen atom has one lone pair of electrons whereas each boron atom has an empty p-orbital. Boron-nitrogen bond (B-N) in borazine is a dative bond which arises from the sideways overlapping between the filled p-orbitals of N atom and empty p-orbitals of B atom. Thus in this way, each boron atom and each nitrogen atom is $s{p^2}$ hybridised in borazine.
Since, benzene is isoelectronic with benzene, both the compounds have aromatic character. Due to greater electronegativity difference in B and N, the electron cloud in the borazine ring is partially delocalised.
Therefore, we concluded that hybridisation of both boron and nitrogen in borazine is $s{p^2}$.
So, the correct answer is “Option C”.
Note: X-ray crystallographic studies shows that bond lengths within the borazine ring are all equivalent at $1.429{A^o}$. The bond angle between the N-B-N atoms is $117.1{A^o}$ and $122.9{A^o}$ between the B-N-B atoms.
Complete Solution :
- Chemical formula of Borazine is ${B_3}{N_3}{H_6}$. Various chemical reactions and electron diffraction studies show that borazine is isoelectronic with the organic molecule i.e., Benzene and hence its structure is same as that of benzene. Like benzene, borazine also has a planar hexagonal structure containing 6 membered rings, in which Boron (B) and Nitrogen (N) atoms are arranged alternately. Because of the similarities between the structures of borazine and benzene, borazine is called as inorganic benzene.
Structure of borazine:

Electronic configuration of Boron, B in ground state: $1{s^2}2{s^2}2{p^1}$.
Electronic configuration of Nitrogen, N in ground state: $1{s^2}2{s^2}2{p^3}$.
Each nitrogen atom has one lone pair of electrons whereas each boron atom has an empty p-orbital. Boron-nitrogen bond (B-N) in borazine is a dative bond which arises from the sideways overlapping between the filled p-orbitals of N atom and empty p-orbitals of B atom. Thus in this way, each boron atom and each nitrogen atom is $s{p^2}$ hybridised in borazine.
Since, benzene is isoelectronic with benzene, both the compounds have aromatic character. Due to greater electronegativity difference in B and N, the electron cloud in the borazine ring is partially delocalised.

Therefore, we concluded that hybridisation of both boron and nitrogen in borazine is $s{p^2}$.
So, the correct answer is “Option C”.
Note: X-ray crystallographic studies shows that bond lengths within the borazine ring are all equivalent at $1.429{A^o}$. The bond angle between the N-B-N atoms is $117.1{A^o}$ and $122.9{A^o}$ between the B-N-B atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




