
How many pi bonds can an atom form?
Answer
555.3k+ views
Hint:Pi bond is formed when the atomic orbitals overlap in such a manner that their axis remains parallel to each other and also their axis is perpendicular to the internuclear axis. The electrons involved in the formation of pi-bonds are called pi electrons.
Complete step-by-step answer:We should remember the fact that a pi bond can only be formed in the molecules which contains double and triple bonds (alkenes and alkynes).
Pi bonds cannot be formed in the compound containing a single bond. This is because whenever a pi bond is formed the compound shows restricted rotation and we know that a single bonded atom rotates freely and does not show restricted rotation.
We should remember that this type of sidewise overlapping always takes place after one of the three p-orbitals has already involved in axial overlapping forming a sigma bond. The rest of two p orbitals are symmetrically placed at right angles to each other and also to the overlapped p orbital. Therefore, these two orbitals are involved in the sidewise overlapping.
Hence, we can say that a pi bond always accompanies a sigma bond or is only formed after one sigma bond is formed in the compound.
Two pi bonds are maximum that can exist between a given pair of atoms.
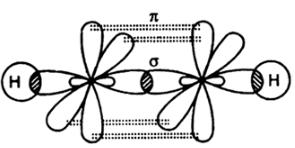
Compound with double bond have one sigma and one pi bond as seen in the case of ethene as shown below:
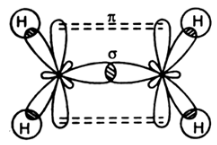
Compounds with triple bond have one sigma and maximum of two pi bonds as seen in the case of ethyne shown below:
Compounds with quadruple bonds are very rare and can only be formed by the transition metals. And they consist of one sigma and two pi bonds and one delta bond.
Note: A pi bond formation shortens the bond distance between the two atoms involved. For example, C-C (sigma bond), C=C double bond (one sigma and one pi) have bond lengths as $154$pm and $134$pm respectively.
Complete step-by-step answer:We should remember the fact that a pi bond can only be formed in the molecules which contains double and triple bonds (alkenes and alkynes).
Pi bonds cannot be formed in the compound containing a single bond. This is because whenever a pi bond is formed the compound shows restricted rotation and we know that a single bonded atom rotates freely and does not show restricted rotation.
We should remember that this type of sidewise overlapping always takes place after one of the three p-orbitals has already involved in axial overlapping forming a sigma bond. The rest of two p orbitals are symmetrically placed at right angles to each other and also to the overlapped p orbital. Therefore, these two orbitals are involved in the sidewise overlapping.
Hence, we can say that a pi bond always accompanies a sigma bond or is only formed after one sigma bond is formed in the compound.
Two pi bonds are maximum that can exist between a given pair of atoms.
Compound with double bond have one sigma and one pi bond as seen in the case of ethene as shown below:

Compounds with triple bond have one sigma and maximum of two pi bonds as seen in the case of ethyne shown below:

Compounds with quadruple bonds are very rare and can only be formed by the transition metals. And they consist of one sigma and two pi bonds and one delta bond.
Note: A pi bond formation shortens the bond distance between the two atoms involved. For example, C-C (sigma bond), C=C double bond (one sigma and one pi) have bond lengths as $154$pm and $134$pm respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




