
How do you read phase diagrams?
Answer
558.9k+ views
Hint: As we know that the phase diagrams are basically the graphical representation of the physical states of a substance such as the solid to liquid phase, liquid to vapour phase and vapour to solid phase provided, the conditions of temperature and pressure are different.
Complete step by step answer:
As we know that the phase transition is the conversion of one state of matter to another like solid to liquid, liquid to vapour and vapour to solid again. We are also aware with the phase diagrams which are the graphical representations of these transitions in phases or states of matter.
In a typical phase diagram, temperature is taken on the x-axis and the pressure is generally taken on the y-axis and when we cross the lines or curves on the phase diagram, a phase change occurs. We can show a graph of general phase diagram as:
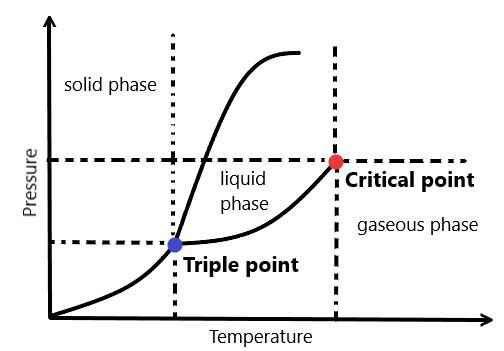
Where the triple point is that point at which all the three phases i.e. solid, liquid and gaseous phase coexist and the critical point is that point at which it becomes difficult to distinguish between a liquid and gaseous phase.
So, a phase diagram is read by plotting the pressure in the atmosphere and temperature in kelvin on the graph in which the labels represent the state of the system in equilibrium and the dotted lines represent the combination of pressure and temperature.
Note:Always remember that a phase diagram helps us to know what phase exists at what temperature and pressure. The temperature and pressure, for most of the substances, related to the triple point generally lies below the standard temperature and pressure and the pressure for the critical point lies above the standard pressure which concludes that at standard pressure as the temperature increases, most substances undergo phase change from solid to liquid to gaseous states.
Complete step by step answer:
As we know that the phase transition is the conversion of one state of matter to another like solid to liquid, liquid to vapour and vapour to solid again. We are also aware with the phase diagrams which are the graphical representations of these transitions in phases or states of matter.
In a typical phase diagram, temperature is taken on the x-axis and the pressure is generally taken on the y-axis and when we cross the lines or curves on the phase diagram, a phase change occurs. We can show a graph of general phase diagram as:

Where the triple point is that point at which all the three phases i.e. solid, liquid and gaseous phase coexist and the critical point is that point at which it becomes difficult to distinguish between a liquid and gaseous phase.
So, a phase diagram is read by plotting the pressure in the atmosphere and temperature in kelvin on the graph in which the labels represent the state of the system in equilibrium and the dotted lines represent the combination of pressure and temperature.
Note:Always remember that a phase diagram helps us to know what phase exists at what temperature and pressure. The temperature and pressure, for most of the substances, related to the triple point generally lies below the standard temperature and pressure and the pressure for the critical point lies above the standard pressure which concludes that at standard pressure as the temperature increases, most substances undergo phase change from solid to liquid to gaseous states.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




