
How do you name imines?
Answer
564.9k+ views
Hint: We know that imine is a chemical compound or a functional group possessing a double bond of carbon and nitrogen. An organic group (R) or a hydrogen (H) atom is attached to the nitrogen atom of the amine.
Complete step by step answer:
Let’s understand imines with the help of an example.
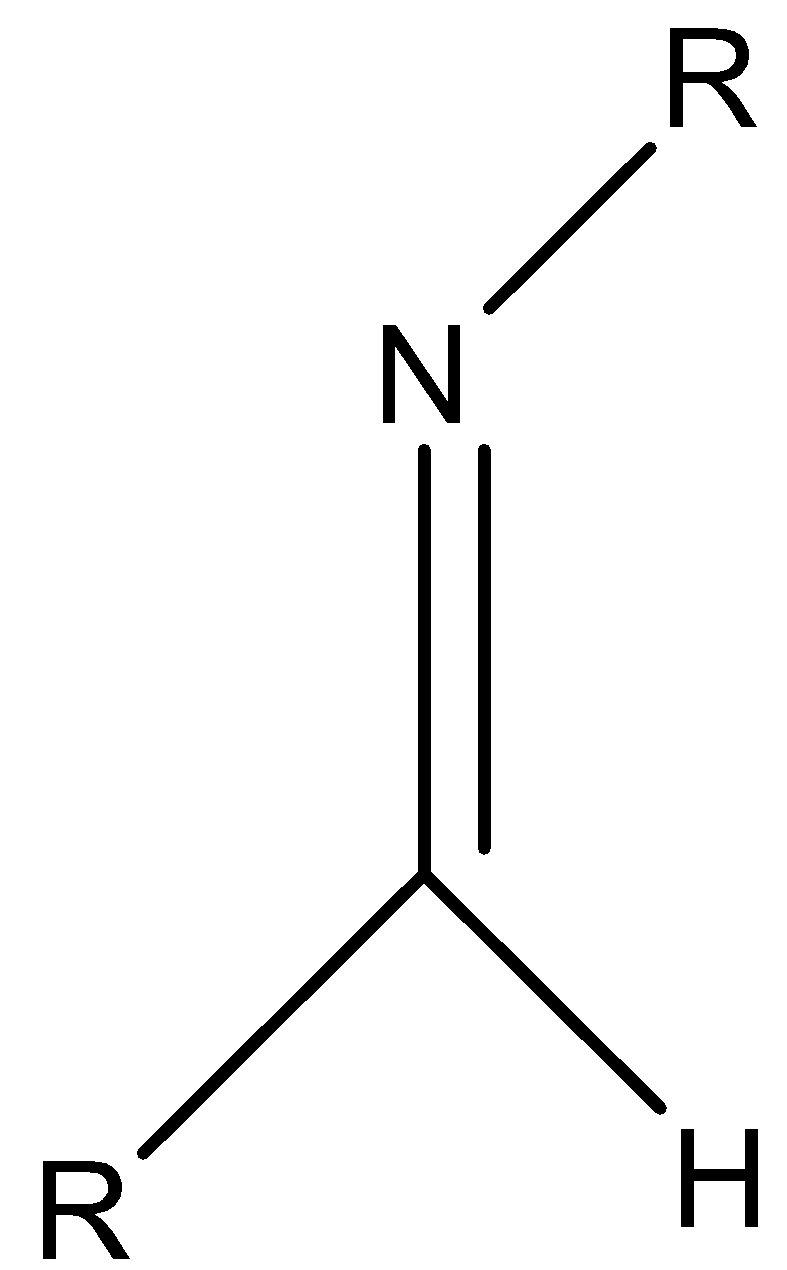
Here, there is a carbon nitrogen double bond present and N atom is attached to an organic group (R).
Let’s discuss the naming of imines. There are different ways of naming mines. In IUPAC naming of imines, we have to name the imines as the alkane substituted imine. That means, we have to add the suffix ‘imine’ to the name of the parent hydrocarbon chain. Let’s understand this rule by taking some examples.
The imine is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$
Here, the parent hydrocarbon chain contains six carbon atoms. So, the name of the imine is Hexan-1-imine.
Another imine is ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_2} = {\text{NH}}$
Here, the parent carbon chain consists of three carbon atoms. So, the name of the imine is propan-2-imine.
Note: Let’s discuss imine derivatives in detail. When aldehydes and ketones react with ammonia formation of imine derivatives takes place. This imine derivative is also called Schiff base. The rate of formation of the imine derivatives is higher near a pH of 5 and its formation drops at lower and higher pH'S.
Another method of naming of imines is naming substitutively as ‘ylidene’ derivatives of parent hydride azane or by replacing the final ‘e’. For example, ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$ imine is named as Hexylideneazane.
Complete step by step answer:
Let’s understand imines with the help of an example.

Here, there is a carbon nitrogen double bond present and N atom is attached to an organic group (R).
Let’s discuss the naming of imines. There are different ways of naming mines. In IUPAC naming of imines, we have to name the imines as the alkane substituted imine. That means, we have to add the suffix ‘imine’ to the name of the parent hydrocarbon chain. Let’s understand this rule by taking some examples.
The imine is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$
Here, the parent hydrocarbon chain contains six carbon atoms. So, the name of the imine is Hexan-1-imine.
Another imine is ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_2} = {\text{NH}}$
Here, the parent carbon chain consists of three carbon atoms. So, the name of the imine is propan-2-imine.
Note: Let’s discuss imine derivatives in detail. When aldehydes and ketones react with ammonia formation of imine derivatives takes place. This imine derivative is also called Schiff base. The rate of formation of the imine derivatives is higher near a pH of 5 and its formation drops at lower and higher pH'S.
Another method of naming of imines is naming substitutively as ‘ylidene’ derivatives of parent hydride azane or by replacing the final ‘e’. For example, ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$ imine is named as Hexylideneazane.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




