
How do you draw ethyl methyl amine?
Answer
537.6k+ views
Hint: If we know the IUPAC nomenclature of the hydrocarbons thoroughly then we could easily decode the structure from the name given. The functional group present is amine group which is $-N{{H}_{2}}$.
Complete step-by-step answer:From the time we study organic chemistry, we study various types of hydrocarbons and its reactions, structure etc.
Here in the question we are supposed to draw the structure of methyl ethyl amine.
For drawing a structure for a molecule, from its name either it should the name and structure of lattice thoroughly or should have the idea on IUPAC nomenclature and try to decode the structural units involved in the reaction.
The method of decoding the structure from the given word is as follows. First we should identify the parent carbon chain which is the longest one and we know that with the number of carbon atoms varies, the prefix changes. And then the substituents given should be identified and the position of the carbon to which the substituent is attached too will also be mentioned in the name of the compound. And in some molecules functional groups are also present and in some multiple bonds too is present.
Now let us talk about the given molecule the name given is ethyl methylamine. The IUPAC nomenclature of the molecule is N-methyl-1- ethanamine.
So the parent carbon skeleton is of ethane i.e. which possesses two C atoms and it is attached to the functional group amine. Then the methyl group which is the substituent specified in the IUPAC naming is attached to the N atom of the amine group.
And the structure of the molecule will be as:
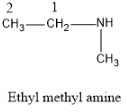
Note:Ethyl methyl amine is a secondary amine, since the amine group is attached to two alkyl chains. And the amine are basic in nature. We could distinguish between the primary, secondary and tertiary amines by carrying out reaction with nitrous acid.
Complete step-by-step answer:From the time we study organic chemistry, we study various types of hydrocarbons and its reactions, structure etc.
Here in the question we are supposed to draw the structure of methyl ethyl amine.
For drawing a structure for a molecule, from its name either it should the name and structure of lattice thoroughly or should have the idea on IUPAC nomenclature and try to decode the structural units involved in the reaction.
The method of decoding the structure from the given word is as follows. First we should identify the parent carbon chain which is the longest one and we know that with the number of carbon atoms varies, the prefix changes. And then the substituents given should be identified and the position of the carbon to which the substituent is attached too will also be mentioned in the name of the compound. And in some molecules functional groups are also present and in some multiple bonds too is present.
Now let us talk about the given molecule the name given is ethyl methylamine. The IUPAC nomenclature of the molecule is N-methyl-1- ethanamine.
So the parent carbon skeleton is of ethane i.e. which possesses two C atoms and it is attached to the functional group amine. Then the methyl group which is the substituent specified in the IUPAC naming is attached to the N atom of the amine group.
And the structure of the molecule will be as:

Note:Ethyl methyl amine is a secondary amine, since the amine group is attached to two alkyl chains. And the amine are basic in nature. We could distinguish between the primary, secondary and tertiary amines by carrying out reaction with nitrous acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




