
Hofmann degradation of m- Bromo benzamide gives :
a.) aniline
b.) m- bromoaniline
c.) bromobenzene
d.) m- Bromo ethyl benzene
Answer
581.1k+ views
Hint: The Hofmann degradation reaction is used to convert primary amide to a primary amine. The general Hofmann’s Bromo amide reaction may be written as -
$RCON{H_2} + B{r_2} + KOH \to RN{H_2}$
Complete answer:
First, let us see what is the Hofmann degradation reaction. This is the reaction to convert primary amide to a primary amine. The general Hofmann’s Bromo amide reaction may be written as -
$RCON{H_2} + B{r_2} + KOH \to RN{H_2}$
So, the reaction of m- Bromo benzamide may be written as -
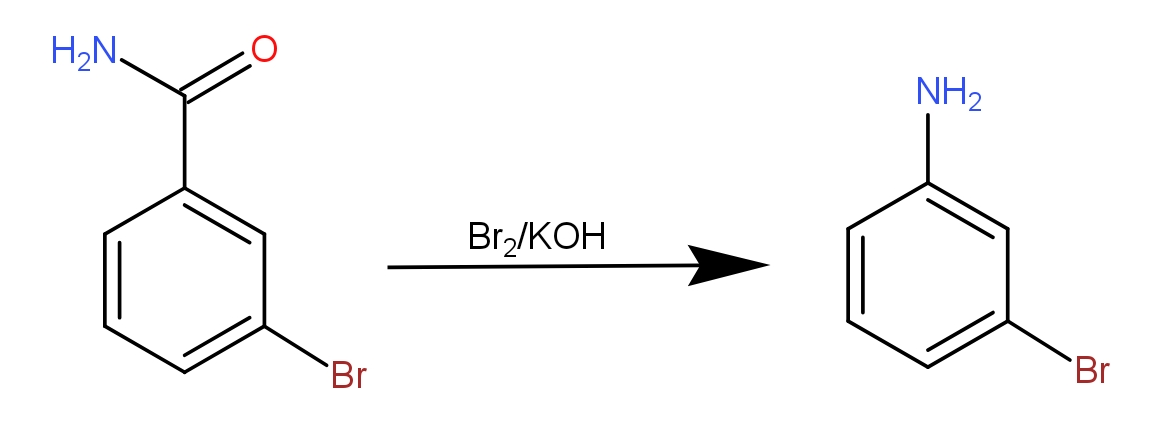
So, the Hofmann degradation of m- Bromo benzamide can give aniline.
Thus, the correct answer is the option a.).
Additional information:
In this reaction, one carbon atom decreases in the product. The reaction of bromine with potassium hydroxide forms potassium hypobromite that converts the primary amide into isocyanate intermediate. This intermediate is then hydrolysed to primary amine and the carbon dioxide is also formed along with the product. The sodium hydroxide can also be used in place of potassium hydroxide. It also reacts in a similar way. The bromine can also be substituted by many other reagents. Such as - Sodium hypochlorite, lead tetraacetate, N- Bromosuccinimide etc. could also be used.
Note: It has various applications. It is used to convert the aliphatic and aromatic both types of amides into amines. It is used in the preparation of anthranilic acid from phthalimide. By using this reaction, the Nicotinic acid can also be converted into 3- Aminopyridine.
$RCON{H_2} + B{r_2} + KOH \to RN{H_2}$
Complete answer:
First, let us see what is the Hofmann degradation reaction. This is the reaction to convert primary amide to a primary amine. The general Hofmann’s Bromo amide reaction may be written as -
$RCON{H_2} + B{r_2} + KOH \to RN{H_2}$
So, the reaction of m- Bromo benzamide may be written as -

So, the Hofmann degradation of m- Bromo benzamide can give aniline.
Thus, the correct answer is the option a.).
Additional information:
In this reaction, one carbon atom decreases in the product. The reaction of bromine with potassium hydroxide forms potassium hypobromite that converts the primary amide into isocyanate intermediate. This intermediate is then hydrolysed to primary amine and the carbon dioxide is also formed along with the product. The sodium hydroxide can also be used in place of potassium hydroxide. It also reacts in a similar way. The bromine can also be substituted by many other reagents. Such as - Sodium hypochlorite, lead tetraacetate, N- Bromosuccinimide etc. could also be used.
Note: It has various applications. It is used to convert the aliphatic and aromatic both types of amides into amines. It is used in the preparation of anthranilic acid from phthalimide. By using this reaction, the Nicotinic acid can also be converted into 3- Aminopyridine.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




