
$\text{HF}$ is a weak acid but $\text{H}\left( \text{FHF} \right)$ is a strong acid. If true enter 1 or 0 for false.
Answer
590.7k+ views
Hint: The acidity of hydrofluoric acid changes with different concentrations and the total extent of interactions of the fluoride ion. At different concentrations, the compounds can form bonds with other molecules as the particles are near. Acidity is the measure of $\left[ {{\text{H}}^{+}} \right]$ ions produced in the solutions.
Complete answer:
Let us discuss the role of concentration in acidity of compounds and find which is strong.
(1) At lower concentrations, hydrogen fluoride is a weak acid but only in dilute aqueous solution. This is because of the strength of the hydrogen–fluorine or $\left( \text{H-F} \right)$ bond and other factor is the ability of $\text{HF,}{{\text{H}}_{2}}\text{O}$ and ${{\text{F}}^{-}}$ ions to form clusters (let’s discuss it a little later). The hydrogen bonding between the two is like
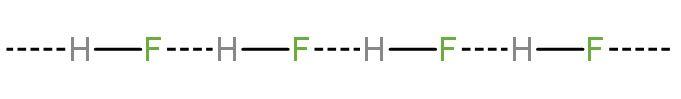
Dilute solutions are weakly acidic with an ionization constant of ${{\text{K}}_{\text{a}}}=6.6\times {{10}^{-4}}$. This weak acidity is attributed to the high $\text{H}-\text{F}$ bond strength that combines with the high dissolution enthalpy of $\text{HF}$ to overpower the more negative enthalpy of hydration of the fluoride ion.
(2) At high concentrations: $\text{HF}$ molecules undergo homo-association to form polyatomic ions like bifluoride, $\text{HF}_{2}^{-}$ and protons like $\text{H}\left( \text{FHF} \right)$ ionizes into ${{\text{H}}^{+}}+\text{FH}{{\text{F}}^{-}}$ which makes it highly acid. The structure is like
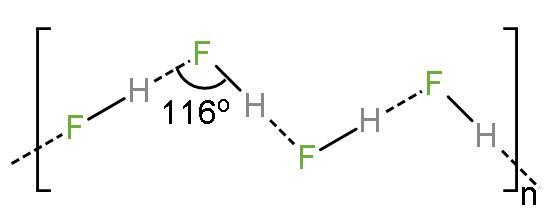
Concentrated solutions of hydrogen fluoride are very strongly acidic. With increasing concentration of $\text{HF}$, the compound starts to form a dimer, thus increasing the concentration of the hydrogen difluoride ions. The reaction is like $3\text{HF}\rightleftharpoons \text{HF}_{2}^{-}+{{\text{H}}_{2}}{{\text{F}}^{+}}$. Forms extensive hydrogen bonding with lone pairs and negative charge present making it highly strong.
The statement in the question is correct so, enter 1.
Additional Information:
Hydrofluoric acid is a weak acid but it is very corrosive. It is used for industrial purposes like metal cleaning and electronics manufacturing. Hydrofluoric acid is also found in home rust removers.
Note:
In thermodynamic terms, $\text{HF}$ solutions are highly non-ideal as with the activity of $\text{HF}$ increasing much more rapidly than its concentration. Not only, temperature but also concentration of an ion affects the acidity of compounds. That’s why, in very dilute solutions only, dissociation of ions is generally ignored.
Complete answer:
Let us discuss the role of concentration in acidity of compounds and find which is strong.
(1) At lower concentrations, hydrogen fluoride is a weak acid but only in dilute aqueous solution. This is because of the strength of the hydrogen–fluorine or $\left( \text{H-F} \right)$ bond and other factor is the ability of $\text{HF,}{{\text{H}}_{2}}\text{O}$ and ${{\text{F}}^{-}}$ ions to form clusters (let’s discuss it a little later). The hydrogen bonding between the two is like

Dilute solutions are weakly acidic with an ionization constant of ${{\text{K}}_{\text{a}}}=6.6\times {{10}^{-4}}$. This weak acidity is attributed to the high $\text{H}-\text{F}$ bond strength that combines with the high dissolution enthalpy of $\text{HF}$ to overpower the more negative enthalpy of hydration of the fluoride ion.
(2) At high concentrations: $\text{HF}$ molecules undergo homo-association to form polyatomic ions like bifluoride, $\text{HF}_{2}^{-}$ and protons like $\text{H}\left( \text{FHF} \right)$ ionizes into ${{\text{H}}^{+}}+\text{FH}{{\text{F}}^{-}}$ which makes it highly acid. The structure is like

Concentrated solutions of hydrogen fluoride are very strongly acidic. With increasing concentration of $\text{HF}$, the compound starts to form a dimer, thus increasing the concentration of the hydrogen difluoride ions. The reaction is like $3\text{HF}\rightleftharpoons \text{HF}_{2}^{-}+{{\text{H}}_{2}}{{\text{F}}^{+}}$. Forms extensive hydrogen bonding with lone pairs and negative charge present making it highly strong.
The statement in the question is correct so, enter 1.
Additional Information:
Hydrofluoric acid is a weak acid but it is very corrosive. It is used for industrial purposes like metal cleaning and electronics manufacturing. Hydrofluoric acid is also found in home rust removers.
Note:
In thermodynamic terms, $\text{HF}$ solutions are highly non-ideal as with the activity of $\text{HF}$ increasing much more rapidly than its concentration. Not only, temperature but also concentration of an ion affects the acidity of compounds. That’s why, in very dilute solutions only, dissociation of ions is generally ignored.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




