
Here, $2,3Dimethylbut-2-ene\xrightarrow[Zn/{{H}_{2}}O]{{{O}_{3}}}?$
What is the final product obtained in the above chemical reaction?
A. Propanal, Propanol
B. Propanone
C. Propanal, Ethanal
D. Propanal, Propanone
Answer
531.9k+ views
Hint: Ozone generally reacts with alkenes and forms ozonides and the formed ozonides undergo reduction reaction with zinc in the presence of water and forms aldehydes or ketones as the products.
Complete answer:
- In the question it is asked to find the final product when 2,3-dimethylbut-2-ene reacts with ozone followed by reduction with zinc in the presence of water.
- The given chemical reaction consists of 2,3-dimethylbut-2-ene.
- We should know the structure of 2,3-dimethylbut-2-ene to complete the given chemical reaction.
- The given chemical reaction contains two steps.
Step-1:
-The structure of 2,3-dimethylbut-2-ene is as follows.
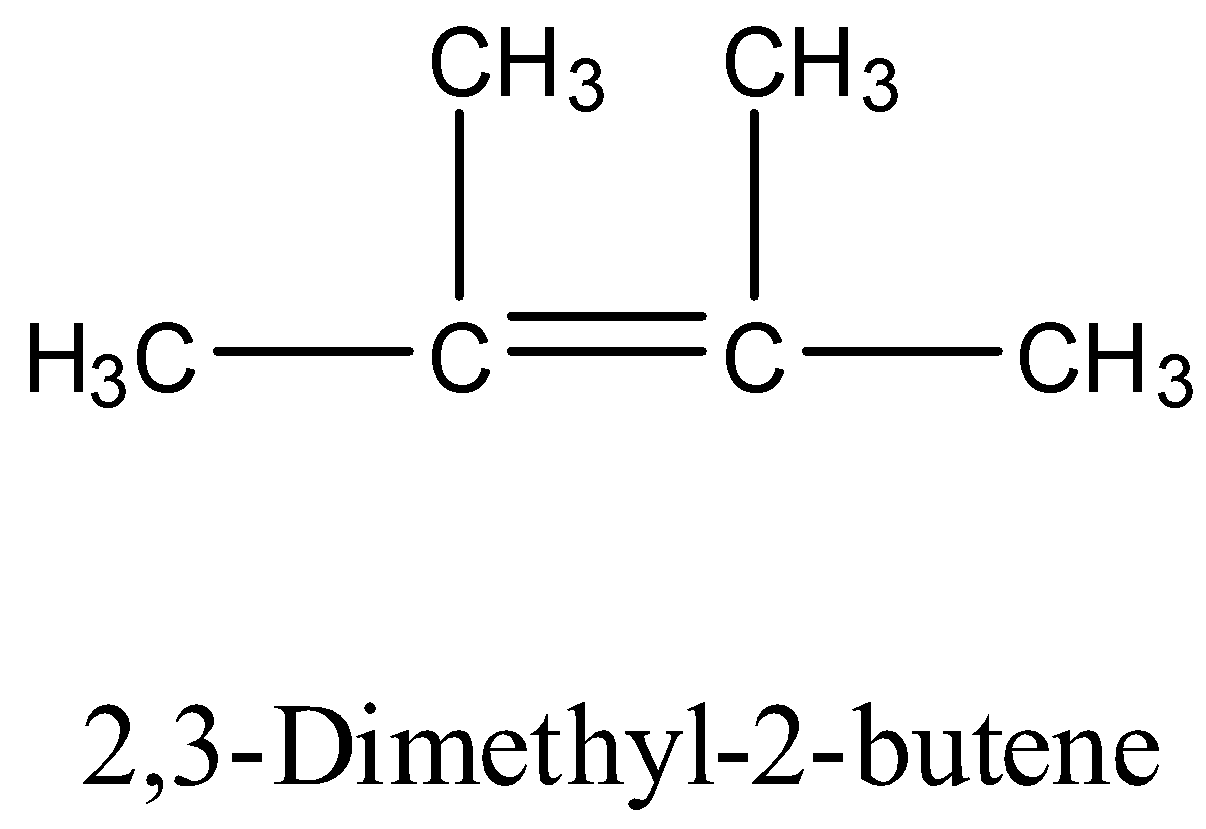
- Now the chemical reaction of 2,3-dimethylbut-2-ene with ozone is as follows.
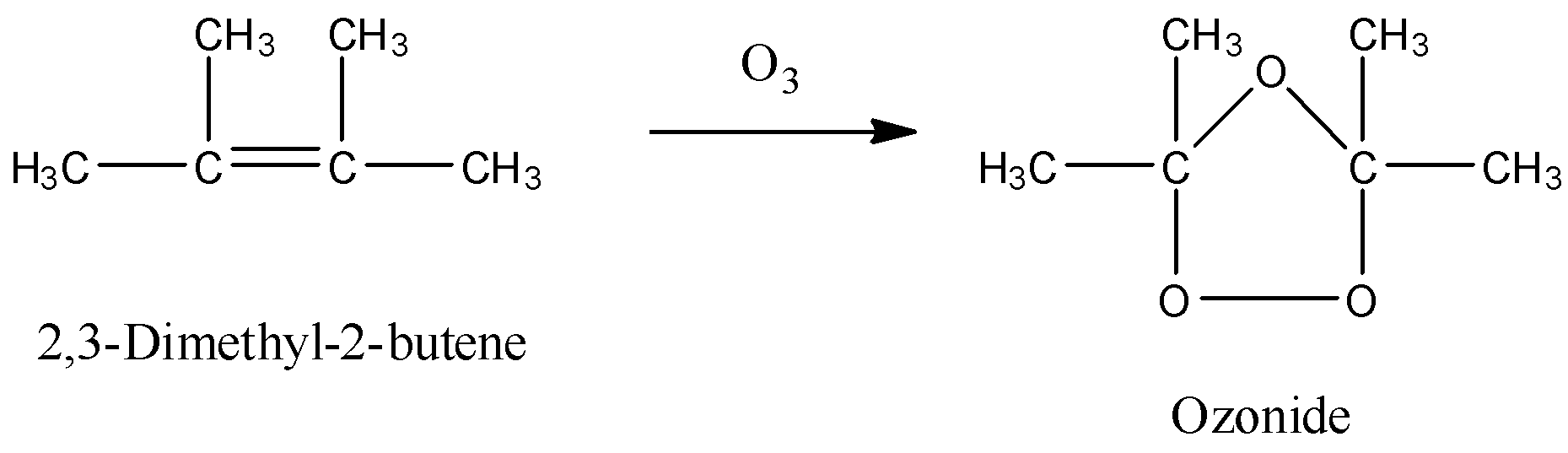
Step-2:
- The formed ozonide is going to react with zinc in the presence of the zinc and forms a reduced compound as the products.
- The chemical reaction of the formed ozonide with zinc is as follows,.
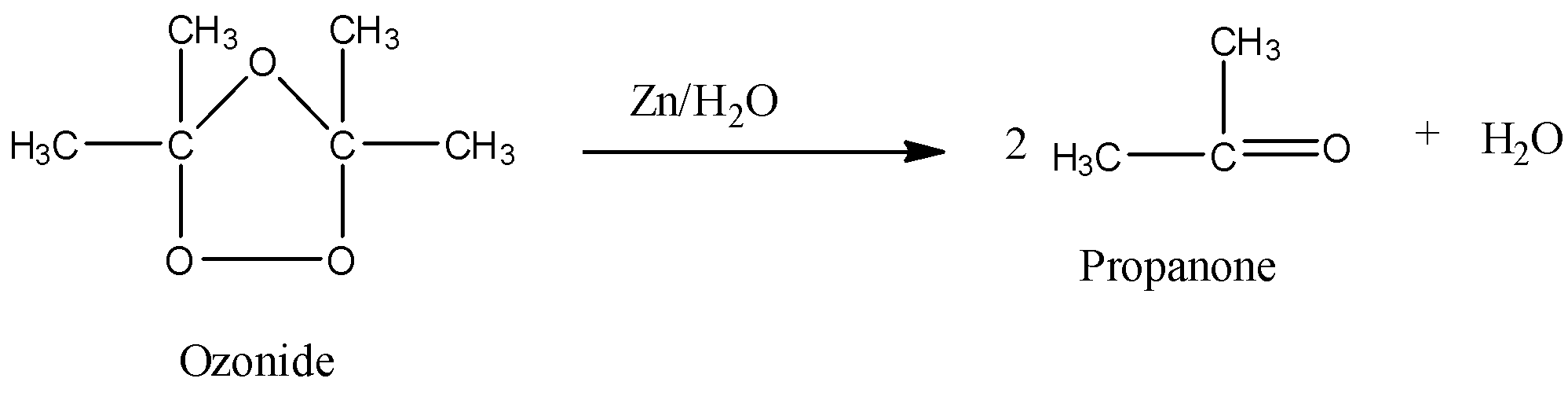
- The products formed in the given chemical reaction are two moles of the propanone and water.
- Therefore 2,3-dimethylbut-2-ene reacts with ozone followed by reduction with zinc in the presence of water and forms two moles of propanone as the product.
So, the correct option B.
Note:
We can detect the presence of the unsaturated hydrocarbons like alkenes and alkynes by using ozone. The ozonide which is formed in the chemical reaction is an intermediate compound and it is not stable in nature.
Complete answer:
- In the question it is asked to find the final product when 2,3-dimethylbut-2-ene reacts with ozone followed by reduction with zinc in the presence of water.
- The given chemical reaction consists of 2,3-dimethylbut-2-ene.
- We should know the structure of 2,3-dimethylbut-2-ene to complete the given chemical reaction.
- The given chemical reaction contains two steps.
Step-1:
-The structure of 2,3-dimethylbut-2-ene is as follows.

- Now the chemical reaction of 2,3-dimethylbut-2-ene with ozone is as follows.

Step-2:
- The formed ozonide is going to react with zinc in the presence of the zinc and forms a reduced compound as the products.
- The chemical reaction of the formed ozonide with zinc is as follows,.

- The products formed in the given chemical reaction are two moles of the propanone and water.
- Therefore 2,3-dimethylbut-2-ene reacts with ozone followed by reduction with zinc in the presence of water and forms two moles of propanone as the product.
So, the correct option B.
Note:
We can detect the presence of the unsaturated hydrocarbons like alkenes and alkynes by using ozone. The ozonide which is formed in the chemical reaction is an intermediate compound and it is not stable in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




