
Here, $\text{1-4 dichlorohexane (1 mole) + NaI (1 mole)}\xrightarrow{Acetone}$ Product of the reaction is:
(a)
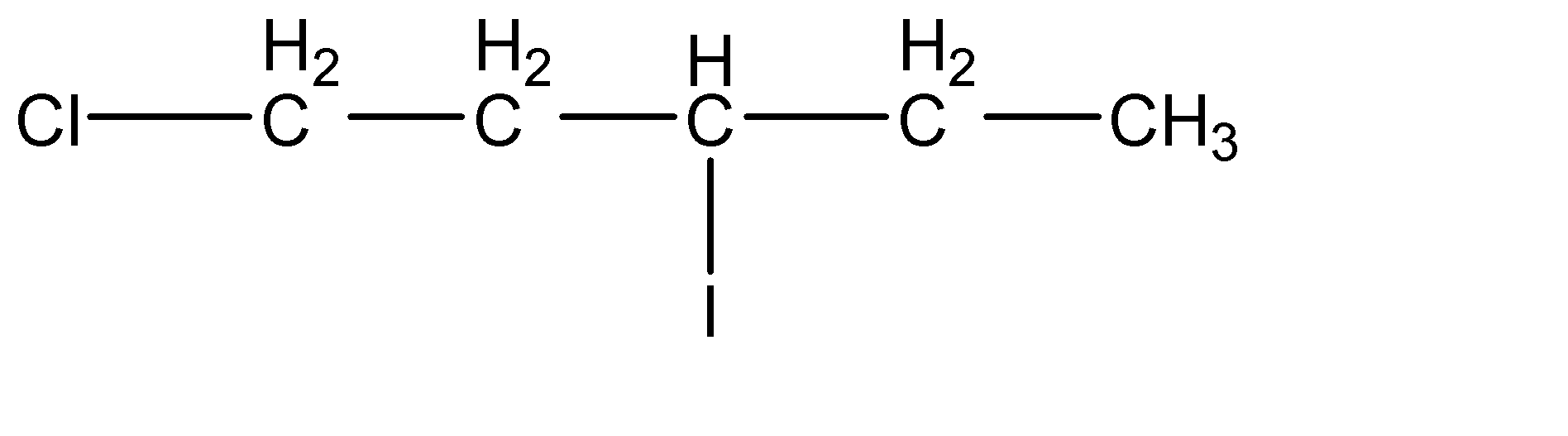
(b)
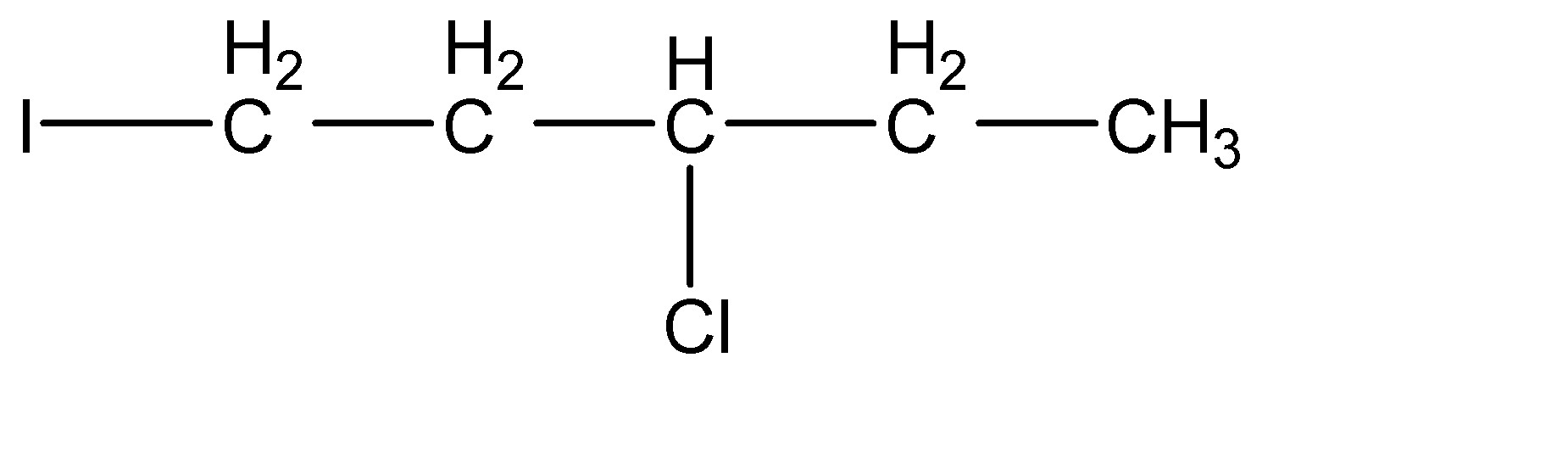
(c)
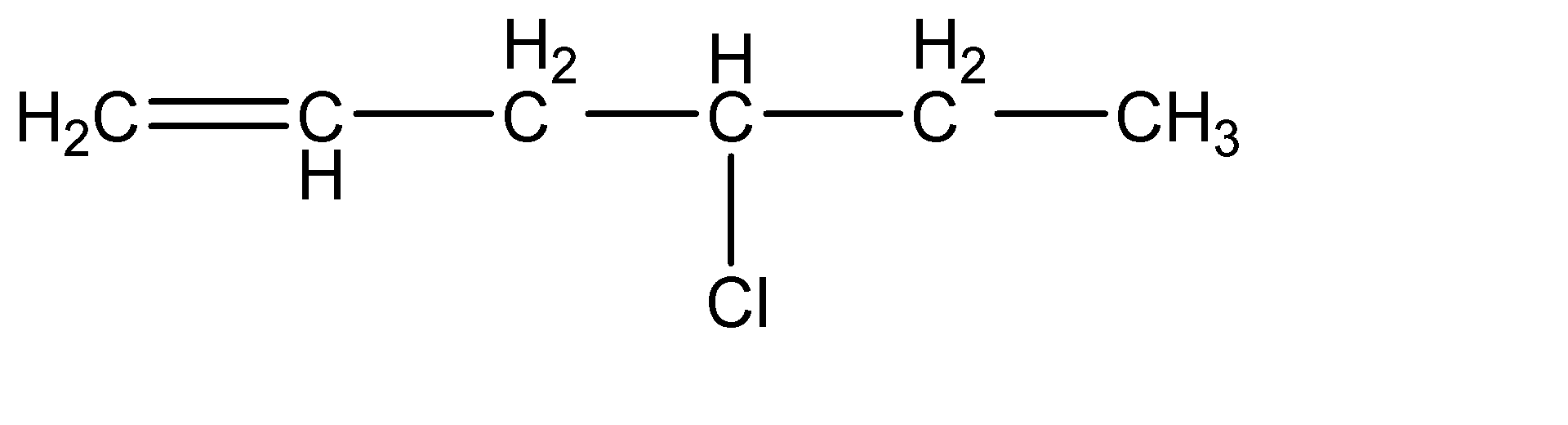
(d)
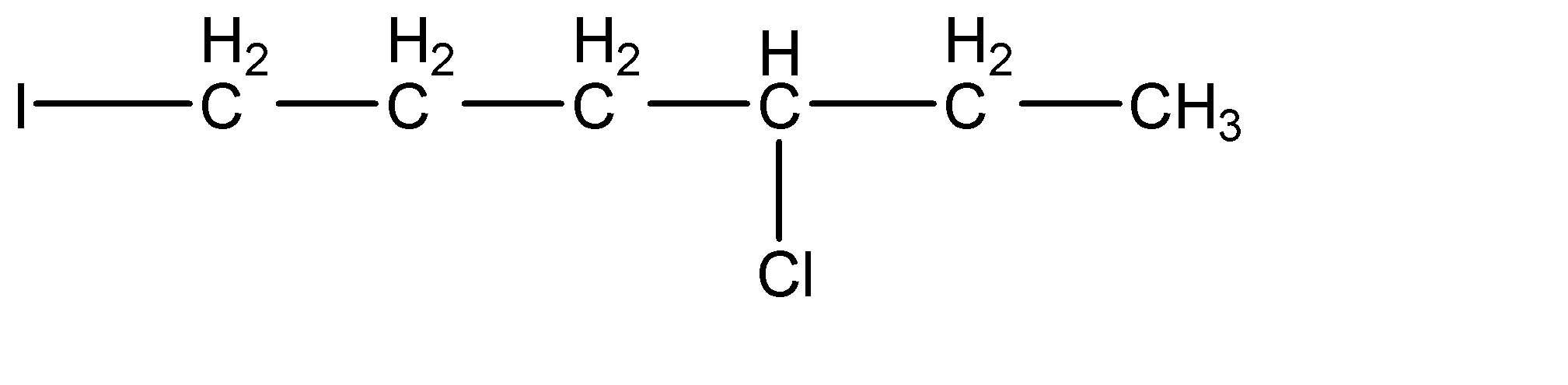




Answer
532.2k+ views
Hint: The above reaction goes through the ${{S}_{N}}2$ reaction mechanism. As we know that the ${{S}_{N}}2$ reaction is a nucleophilic substitution reaction in which a bond is broken and another is formed simultaneously. Two reacting species are involved in the rate determining step of the reaction that takes place.
Complete answer:
Let us first discuss about ${{S}_{N}}2$ reaction mechanism as follows:-
-${{S}_{N}}2$ reaction mechanism: It is a nucleophilic substitution reaction in which a bond is broken and another is formed simultaneously. Two reacting species are involved in the rate determining step of the reaction that takes place. It is also known as bimolecular nucleophilic substitution, associative substitution, and interchange mechanism.
-When sodium iodide (NaI) is mixed in acetone, it gets completely soluble in it and hence breaks into $N{{a}^{+}}$ and ${{I}^{-}}$ ions where iodide ion act as a nucleophile during the reaction.
-The reaction takes place as follows:-
-As we can see that iodide ion (${{I}^{-}}$) acts as a nucleophile and attacks at the carbon site where the leaving group i.e., chloride (-Cl) group is attached. They form a transition state where Chlorine atom is leaving and iodine is attaching itself to the carbon and at the end we obtain 1-iodo-4-chlorohexane.
- After this the chloride ion interacts with sodium ion and forms sodium chloride (NaCl).
-Also iodide ion (${{I}^{-}}$) attacks the chlorine of carbon-1 than chlorine at carbon-2 because ${{S}_{N}}2$ mechanism prefers primary site over secondary site due to hindrance reasons.
Hence, the product of the reaction is (d) .
Note:
-As sodium iodide is more soluble in acetone as compared to sodium chloride which is least soluble in it, so we will also obtain NaCl as precipitate during the reaction.
-${{S}_{N}}2$ reaction mechanism always prefers less hindered sites over more hindered sites.
Complete answer:
Let us first discuss about ${{S}_{N}}2$ reaction mechanism as follows:-
-${{S}_{N}}2$ reaction mechanism: It is a nucleophilic substitution reaction in which a bond is broken and another is formed simultaneously. Two reacting species are involved in the rate determining step of the reaction that takes place. It is also known as bimolecular nucleophilic substitution, associative substitution, and interchange mechanism.
-When sodium iodide (NaI) is mixed in acetone, it gets completely soluble in it and hence breaks into $N{{a}^{+}}$ and ${{I}^{-}}$ ions where iodide ion act as a nucleophile during the reaction.
-The reaction takes place as follows:-
-As we can see that iodide ion (${{I}^{-}}$) acts as a nucleophile and attacks at the carbon site where the leaving group i.e., chloride (-Cl) group is attached. They form a transition state where Chlorine atom is leaving and iodine is attaching itself to the carbon and at the end we obtain 1-iodo-4-chlorohexane.
- After this the chloride ion interacts with sodium ion and forms sodium chloride (NaCl).
-Also iodide ion (${{I}^{-}}$) attacks the chlorine of carbon-1 than chlorine at carbon-2 because ${{S}_{N}}2$ mechanism prefers primary site over secondary site due to hindrance reasons.
Hence, the product of the reaction is (d) .
Note:
-As sodium iodide is more soluble in acetone as compared to sodium chloride which is least soluble in it, so we will also obtain NaCl as precipitate during the reaction.
-${{S}_{N}}2$ reaction mechanism always prefers less hindered sites over more hindered sites.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




