
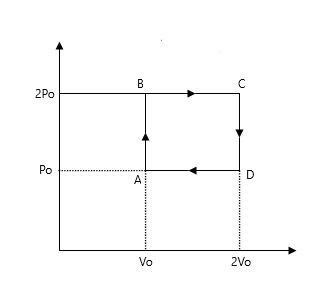
Helium gas goes through a cycle ABCDA (consisting of two isochoric and two isobaric lines) as shown in the figure. Calculate the approximate efficiency of the cycle. (Assume the gas to be close to ideal gas).

Answer
569.7k+ views
Hint: We have to calculate the efficiency and efficiency is the ratio of work done and heat given or taken
$\eta =\dfrac{W}{Q}$
Further, the heat in this process can find out with the help of Molar heat capacities. Molar heat capacity is the quantity of heat required to raise the temperature of 1 mole of a substance by 1 degree. Efficiency doesn’t have any units.
Complete Solution :
If we look at the process AB then there is an increase of pressure in it and the process BC, the volume is increasing, therefore the temperature is increasing.
Now, you may ask that how the temperature is increasing then, we all know if the volume is constant then pressure is directly proportional to the temperature in AB path and when pressure is constant in BC then the volume is directly proportional to the temperature.
The increase in temperature can be calculated by:
$Q=n{{\left( {{C}_{v}}\Delta T \right)}_{AB}}+n{{\left( {{C}_{p}}\Delta T \right)}_{BC}}$ ${{C}_{P}}=\dfrac{3}{2}R$
$\Rightarrow Q=\dfrac{3}{2}{{\left( nR\Delta T \right)}_{AB}}+\dfrac{5}{2}{{\left( nR\Delta T \right)}_{BC}}$ ${{C}_{V}}=\dfrac{5}{2}R$
$\Rightarrow Q=\dfrac{3}{2}\left( {{P}_{0}}{{V}_{0}} \right)+\dfrac{5}{2}\left( 2{{P}_{0}}{{V}_{0}} \right)$ $PV=nRT$ $\left( n = 2for{{C}_{P}} \right)$
$\therefore Q=\dfrac{13}{2}{{P}_{0}}{{V}_{0}}$
We have found the heat required in the process, now we will find the work done required for it.
W=Area under the P-V graph
$W=P\left( 2{{V}_{0}}-{{V}_{0}} \right)$
$\Rightarrow W={{P}_{0}}{{V}_{0}}$
Now, we have both the work and heat with us, let us put it in the formula of efficiency.
$\eta =\dfrac{W}{Q}$
Putting the values
$\eta =\dfrac{{{P}_{0}}{{V}_{0}}}{\dfrac{13}{2}{{P}_{0}}{{V}_{0}}}$
$\Rightarrow \eta =\dfrac{2}{13}=0.0154$
$\therefore \eta = 15.4%$
Therefore, the efficiency of the process is 15.4%
Note: The process which is occurring above in the CD and DA part is not considered. As in two of the processes, both the volume and temperature are decreasing and due to the negative sign later in the process, the value would have become zero.
$\eta =\dfrac{W}{Q}$
Further, the heat in this process can find out with the help of Molar heat capacities. Molar heat capacity is the quantity of heat required to raise the temperature of 1 mole of a substance by 1 degree. Efficiency doesn’t have any units.
Complete Solution :
If we look at the process AB then there is an increase of pressure in it and the process BC, the volume is increasing, therefore the temperature is increasing.
Now, you may ask that how the temperature is increasing then, we all know if the volume is constant then pressure is directly proportional to the temperature in AB path and when pressure is constant in BC then the volume is directly proportional to the temperature.
The increase in temperature can be calculated by:
$Q=n{{\left( {{C}_{v}}\Delta T \right)}_{AB}}+n{{\left( {{C}_{p}}\Delta T \right)}_{BC}}$ ${{C}_{P}}=\dfrac{3}{2}R$
$\Rightarrow Q=\dfrac{3}{2}{{\left( nR\Delta T \right)}_{AB}}+\dfrac{5}{2}{{\left( nR\Delta T \right)}_{BC}}$ ${{C}_{V}}=\dfrac{5}{2}R$
$\Rightarrow Q=\dfrac{3}{2}\left( {{P}_{0}}{{V}_{0}} \right)+\dfrac{5}{2}\left( 2{{P}_{0}}{{V}_{0}} \right)$ $PV=nRT$ $\left( n = 2for{{C}_{P}} \right)$
$\therefore Q=\dfrac{13}{2}{{P}_{0}}{{V}_{0}}$
We have found the heat required in the process, now we will find the work done required for it.
W=Area under the P-V graph
$W=P\left( 2{{V}_{0}}-{{V}_{0}} \right)$
$\Rightarrow W={{P}_{0}}{{V}_{0}}$
Now, we have both the work and heat with us, let us put it in the formula of efficiency.
$\eta =\dfrac{W}{Q}$
Putting the values
$\eta =\dfrac{{{P}_{0}}{{V}_{0}}}{\dfrac{13}{2}{{P}_{0}}{{V}_{0}}}$
$\Rightarrow \eta =\dfrac{2}{13}=0.0154$
$\therefore \eta = 15.4%$
Therefore, the efficiency of the process is 15.4%
Note: The process which is occurring above in the CD and DA part is not considered. As in two of the processes, both the volume and temperature are decreasing and due to the negative sign later in the process, the value would have become zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




