
Helium atoms have an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer
591.9k+ views
Hint: Atom is the smallest fundamental particle of matter. The subatomic particles of an atom are protons, neutrons and electrons. Atomic mass is the sum of the number of protons and neutrons.
Formula Used:
Atomic mass = Number of protons + Number of neutrons
Complete answer:
Protons are positively charged particles, neutrons are neutral particles and electrons are negatively charged particles. Protons and neutrons present in the nucleus of an atom while electrons revolve around the nucleus.
Atomic mass indicates the total number of protons and neutrons present in the nucleus of an atom.
Calculate the number of neutrons present in a helium atom using the following formula.
Atomic mass = Number of protons + Number of neutrons
We have given atomic mass and number of protons of helium.
Atomic mass of helium = 4 u
Number of protons = 2
Now, substitute the given value in the atomic mass formula and calculate the number of neutrons as follows:
4 = 2 + Number of neutrons
Number of neutrons = 4 - 2
Number of neutrons = 2
Hence, the number of neutrons in the helium atom is 2.
Additional Information An atom is the smallest individual particle of an element that takes part in the chemical reaction. Atoms consist of three basic subatomic particles. They are protons, neutrons and electrons. The atomic number of an atom indicates the number of protons present in an atom while the atomic mass number indicates the total number of protons and neutrons. In a neutral atom the number of electrons is always equal to the number of protons.
The helium atom contains 2 protons, 2 neutrons and 2 electrons so the structure of the helium atom is
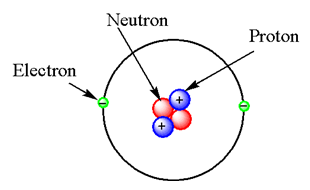
Note: Atomic number is the number of protons present in the nucleus of an atom. Atomic mass is the total number of protons and neutrons present in the nucleus of an atom. The numbers of neutrons are always less than the atomic mass number.
Formula Used:
Atomic mass = Number of protons + Number of neutrons
Complete answer:
Protons are positively charged particles, neutrons are neutral particles and electrons are negatively charged particles. Protons and neutrons present in the nucleus of an atom while electrons revolve around the nucleus.
Atomic mass indicates the total number of protons and neutrons present in the nucleus of an atom.
Calculate the number of neutrons present in a helium atom using the following formula.
Atomic mass = Number of protons + Number of neutrons
We have given atomic mass and number of protons of helium.
Atomic mass of helium = 4 u
Number of protons = 2
Now, substitute the given value in the atomic mass formula and calculate the number of neutrons as follows:
4 = 2 + Number of neutrons
Number of neutrons = 4 - 2
Number of neutrons = 2
Hence, the number of neutrons in the helium atom is 2.
Additional Information An atom is the smallest individual particle of an element that takes part in the chemical reaction. Atoms consist of three basic subatomic particles. They are protons, neutrons and electrons. The atomic number of an atom indicates the number of protons present in an atom while the atomic mass number indicates the total number of protons and neutrons. In a neutral atom the number of electrons is always equal to the number of protons.
The helium atom contains 2 protons, 2 neutrons and 2 electrons so the structure of the helium atom is

Note: Atomic number is the number of protons present in the nucleus of an atom. Atomic mass is the total number of protons and neutrons present in the nucleus of an atom. The numbers of neutrons are always less than the atomic mass number.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




