
What happens when two or more drops of iodine solution fall on starch substance?
Answer
579k+ views
Hint: Iodine in the presence of starch shows blue-black colouration due to the formation of starch-iodine complex in the form of helices. This property of Iodine is used in testing the presence of starch.
Complete answer:
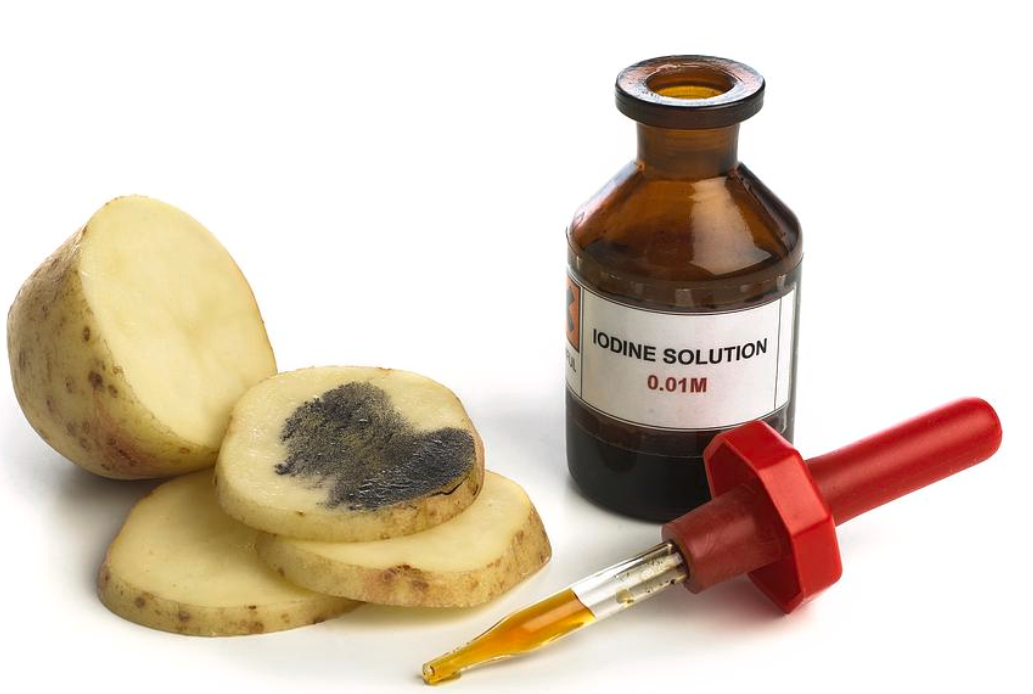
When two or more drops of iodine solution fall on starch substance, we observe a blue-black colouration. Iodine is originally yellow in colour. In the presence of starch, this yellow colour changes to blue-black colour due to the formation of starch-iodine complex that imparts the blue colour. This property of iodine is often used in laboratories to detect the presence of starch. When a colour change doesn’t occur, this test also indicates the completion of hydrolysis of starch.
Additional information:
Originally, iodine is yellow in colour. The principle of the reaction is based on the hydrolysis of starch. The reaction is due to the formation of polyiodide chains from the reaction between starch and iodine. The amylose present in the starch forms helices where iodine molecules assemble, forming a dark blue or black colour. When starch is broken down or hydrolysed into smaller carbohydrate units, the blue-black colour is not generated. When the hydrolysis is complete, this blue-black colouration is not observed. This interaction of starch and triiodide is the basis of iodometry.
Note: In starch hydrolysis test, iodine is used as an indicator. It is often used in industries to detect the amylase activity of a particular microorganism in hydrolysis of starch. No change in iodine colour is observed if the amylase activity of the microorganism is strong enough to hydrolyse the entire starch. In the presence of amylase, starch is hydrolysed into maltose and glucose.
Complete answer:
When two or more drops of iodine solution fall on starch substance, we observe a blue-black colouration. Iodine is originally yellow in colour. In the presence of starch, this yellow colour changes to blue-black colour due to the formation of starch-iodine complex that imparts the blue colour. This property of iodine is often used in laboratories to detect the presence of starch. When a colour change doesn’t occur, this test also indicates the completion of hydrolysis of starch.

Additional information:
Originally, iodine is yellow in colour. The principle of the reaction is based on the hydrolysis of starch. The reaction is due to the formation of polyiodide chains from the reaction between starch and iodine. The amylose present in the starch forms helices where iodine molecules assemble, forming a dark blue or black colour. When starch is broken down or hydrolysed into smaller carbohydrate units, the blue-black colour is not generated. When the hydrolysis is complete, this blue-black colouration is not observed. This interaction of starch and triiodide is the basis of iodometry.
Note: In starch hydrolysis test, iodine is used as an indicator. It is often used in industries to detect the amylase activity of a particular microorganism in hydrolysis of starch. No change in iodine colour is observed if the amylase activity of the microorganism is strong enough to hydrolyse the entire starch. In the presence of amylase, starch is hydrolysed into maltose and glucose.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




