
What happens when sodium hydroxide reacts with sulphuric acid?
Answer
509.7k+ views
Hint: Sodium hydroxide is the hydroxide of an alkali metal and is therefore basic in nature and sulphuric acid is a strong diprotic acid. Acids and bases react with each other to give salt and water as a by -product. The salt can be acidic, basic or neutral.
Complete answer:
The reaction that takes place between an acid and a base to give out salt and water as the final products is known as a neutralization reaction.
The salt formation utilizes one or more cations from the base and anions from the acid resulting in an ionic compound. The charge or valency of the cation in base and that of the anion in acid is used to determine the chemical formula of the salt formed.
Sodium hydroxide is a strong base that dissociates to give sodium cation and hydroxide anion. Sulphuric acid is a diprotic acid which means it releases two moles of hydrogen ions per more of the acid. Apart from giving hydrogen ions, it also gives sulphate anions. Thus two moles of univalent sodium ions combine with one mole of divalent sulphate ions to give sodium sulphate as the salt. Since the acidic strength of sulphuric acid and the basic strength of sodium hydroxide is nearly the same, sodium sulphate is a neutral salt. The hydroxide and hydrogens ions combine to give water.
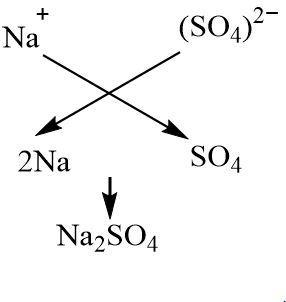
The neutralization reaction between sodium hydroxide and sulphuric acid can be represented by the following chemical equation:
$ NaOH(aq) + {H_2}S{O_4}(aq) \to N{a_2}S{O_4}(aq) + {H_2}O(l) $
Note:
Neutralization reaction is a highly exothermic reaction which means that a lot of heat is evolved in the process that leads to a sudden rise in the temperature of the container in which the reaction is being carried out, especially in the case of neutralization between a strong acid and strong base.
Complete answer:
The reaction that takes place between an acid and a base to give out salt and water as the final products is known as a neutralization reaction.
The salt formation utilizes one or more cations from the base and anions from the acid resulting in an ionic compound. The charge or valency of the cation in base and that of the anion in acid is used to determine the chemical formula of the salt formed.
Sodium hydroxide is a strong base that dissociates to give sodium cation and hydroxide anion. Sulphuric acid is a diprotic acid which means it releases two moles of hydrogen ions per more of the acid. Apart from giving hydrogen ions, it also gives sulphate anions. Thus two moles of univalent sodium ions combine with one mole of divalent sulphate ions to give sodium sulphate as the salt. Since the acidic strength of sulphuric acid and the basic strength of sodium hydroxide is nearly the same, sodium sulphate is a neutral salt. The hydroxide and hydrogens ions combine to give water.

The neutralization reaction between sodium hydroxide and sulphuric acid can be represented by the following chemical equation:
$ NaOH(aq) + {H_2}S{O_4}(aq) \to N{a_2}S{O_4}(aq) + {H_2}O(l) $
Note:
Neutralization reaction is a highly exothermic reaction which means that a lot of heat is evolved in the process that leads to a sudden rise in the temperature of the container in which the reaction is being carried out, especially in the case of neutralization between a strong acid and strong base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




