
What happens when glycerol is heated with $KHS{{O}_{4}}$?
Answer
523.5k+ views
Hint: Glycerol is a type of poly hydroxyl molecule called as a triol that has 3 alcohol functional groups with a formula, ${{C}_{3}}{{H}_{8}}{{O}_{3}}$. Potassium hydrogen sulphate is $KHS{{O}_{4}}$ that is used as an acid based catalyst for organic reactions. The heating of glycerol with potassium bisulphate can result in a dehydration reaction.
Complete answer:
A glycerol is a poly hydroxyl organic compound that has 3 alcoholic groups (OH groups) also called a triol. Glycerol is heated in the presence of potassium bisulphate that results in an acid catalyzed dehydration reaction, as potassium bisulphate acts as an acid in organic reactions. This acid catalyzed dehydration results in the removal of two units of water molecules from the glycerol that forms a compound having an unsaturated group and an aldehyde group that is $\alpha ,\beta $- unsaturated aldehyde also called as acryl aldehyde.
The reaction is as follows:
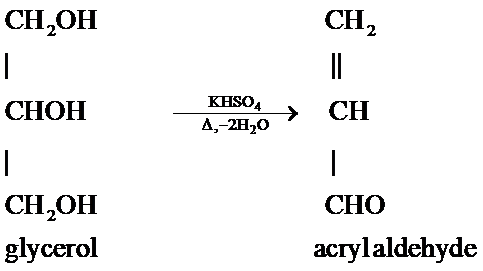
Hence, when glycerol is heated with $KHS{{O}_{4}}$ then a dehydration reaction occurs by the removal of water that results in the formation of an $\alpha ,\beta $- unsaturated aldehyde also called as acryl aldehyde.
Note:
Glycerol is a polyol compound that is a common by-product in the soap industry. Glycerol has moisturizing properties and is used in skin softening. On treating it with potassium bisulphate, acrolein is formed that is $\alpha ,\beta $- unsaturated aldehyde. Acrolein is used in synthesis of acrylic acid. Also acrolein is formed when any oil or fat is subjected to heat.
Complete answer:
A glycerol is a poly hydroxyl organic compound that has 3 alcoholic groups (OH groups) also called a triol. Glycerol is heated in the presence of potassium bisulphate that results in an acid catalyzed dehydration reaction, as potassium bisulphate acts as an acid in organic reactions. This acid catalyzed dehydration results in the removal of two units of water molecules from the glycerol that forms a compound having an unsaturated group and an aldehyde group that is $\alpha ,\beta $- unsaturated aldehyde also called as acryl aldehyde.
The reaction is as follows:
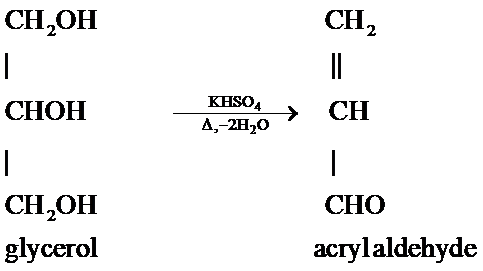
Hence, when glycerol is heated with $KHS{{O}_{4}}$ then a dehydration reaction occurs by the removal of water that results in the formation of an $\alpha ,\beta $- unsaturated aldehyde also called as acryl aldehyde.
Note:
Glycerol is a polyol compound that is a common by-product in the soap industry. Glycerol has moisturizing properties and is used in skin softening. On treating it with potassium bisulphate, acrolein is formed that is $\alpha ,\beta $- unsaturated aldehyde. Acrolein is used in synthesis of acrylic acid. Also acrolein is formed when any oil or fat is subjected to heat.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




