
What happens when acetaldehyde Is treated with phenylhydrazine? Give a balanced chemical equation?
Answer
504.9k+ views
Hint: So, the acetaldehyde is also known as the ‘Ethanal’ because it is the aldehyde produced from the acetic acid by reduction of the carboxyl group. This acetaldehyde undergoes a reaction with the phenylhydrazine and readily forms a product with the release of the water molecule.
Complete answer: Acetaldehyde is one of the most abundant carcinogens present in the smoke.
On the other hand phenylhydrazine is present in nature or exists as yellow crystals or in the form of yellow liquid, which is tremendously used as a chemical in various reactions.
This phenylhydrazine is also found naturally in many edible mushrooms.
Now we have basic knowledge regarding both of the compound that has to undergo reaction, so let’s proceeded with the equation:
So, when acetaldehyde is treated with phenylhydrazine, the Nitrogen atom of the phenylhydrazene serves as a nucleophile and attacks the electrophilic carbon of the acetaldehyde and removes water to give acetaldehyde phenylhydrazone.
Important point to note is that in this reaction, the water molecule is released..
Also, for a better understanding let’s have a look at the equation diagrammatically
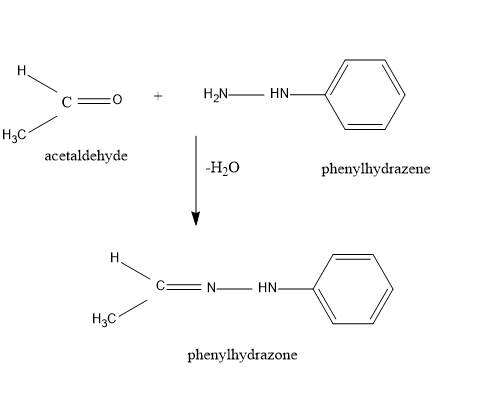
So, from the above equation, the reaction between phenylhydrazine and acetaldehyde is crystal clear.
Thus, when acetaldehyde Is treated with phenylhydrazine it gives acetaldehyde phenylhydrazone.
Note:
Phenylhydrazine is used to form phenylhydrazones of natural mixtures of simple sugars in order to render the differing sugars easily separable from each other. This molecule is also used to induce acute hemolytic anemia in animal models.
Complete answer: Acetaldehyde is one of the most abundant carcinogens present in the smoke.
On the other hand phenylhydrazine is present in nature or exists as yellow crystals or in the form of yellow liquid, which is tremendously used as a chemical in various reactions.
This phenylhydrazine is also found naturally in many edible mushrooms.
Now we have basic knowledge regarding both of the compound that has to undergo reaction, so let’s proceeded with the equation:
So, when acetaldehyde is treated with phenylhydrazine, the Nitrogen atom of the phenylhydrazene serves as a nucleophile and attacks the electrophilic carbon of the acetaldehyde and removes water to give acetaldehyde phenylhydrazone.
Important point to note is that in this reaction, the water molecule is released..
Also, for a better understanding let’s have a look at the equation diagrammatically

So, from the above equation, the reaction between phenylhydrazine and acetaldehyde is crystal clear.
Thus, when acetaldehyde Is treated with phenylhydrazine it gives acetaldehyde phenylhydrazone.
Note:
Phenylhydrazine is used to form phenylhydrazones of natural mixtures of simple sugars in order to render the differing sugars easily separable from each other. This molecule is also used to induce acute hemolytic anemia in animal models.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




