
What happens if you mix acetone and water?
Answer
513.6k+ views
Hint: We need to first shortlist all the possibilities of what could be the outcome when we mix two different types of liquids/chemicals. This can have different results based on the solubility between two, the chemical reaction that can take place, resulting in heat absorption or heat release (i.e. endothermic or exothermic reactions), etc.
Complete answer:
The chemical formula of acetone is $C{H_3}COC{H_3}$ . If we see the structure of acetone, it is of the form:
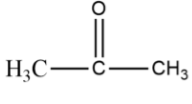
Now, if we look at a water molecule, it has the molecular formula ${H_2}O$ . If we look at its structure, it is of the form:
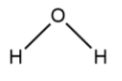
When we add acetone to water, in that case, water acts as a solvent and acetone acts as a solute. On the other hand, if we add water in acetone, we realize that now acetone will act as a solvent and water will act as a solute. In both the cases, the reaction wouldn’t be any complex and would not form any different chemical product.
Although, there will be slight hydrogen bonding that will take place in the container in which we have mixed acetone and water. The H-bonding is of the form:
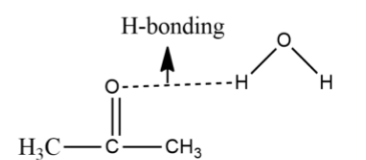
This hydrogen bonding is weak. Also, the mixture of water and acetone will be homogeneous and no two layers will be formed.
Note:
It is generally seen that the mixture of two liquids gives rise to an azeotrope mixture. However, in case of acetone-water mixture, the homogeneous solution will not have any azeotropic formation and the mixture can be easily separated by distillation process, since both have big differences in their boiling point.
Complete answer:
The chemical formula of acetone is $C{H_3}COC{H_3}$ . If we see the structure of acetone, it is of the form:

Now, if we look at a water molecule, it has the molecular formula ${H_2}O$ . If we look at its structure, it is of the form:

When we add acetone to water, in that case, water acts as a solvent and acetone acts as a solute. On the other hand, if we add water in acetone, we realize that now acetone will act as a solvent and water will act as a solute. In both the cases, the reaction wouldn’t be any complex and would not form any different chemical product.
Although, there will be slight hydrogen bonding that will take place in the container in which we have mixed acetone and water. The H-bonding is of the form:

This hydrogen bonding is weak. Also, the mixture of water and acetone will be homogeneous and no two layers will be formed.
Note:
It is generally seen that the mixture of two liquids gives rise to an azeotrope mixture. However, in case of acetone-water mixture, the homogeneous solution will not have any azeotropic formation and the mixture can be easily separated by distillation process, since both have big differences in their boiling point.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




