
Halogenation of alkene using $ B{r_2}/CC{l_4} $ is syn addition but not anti addition. If this is true enter1, else enter 0.
Answer
546.3k+ views
Hint: When halogen is added on the same side of the alkene, it undergoes syn addition. When halogen is added on the opposite side of the alkene, it undergoes anti addition. If we know this basic, solution to the question becomes easy to handle.
Complete step by step answer:
Elements like chlorine and bromine are $ {e^ - } $ loving species. They are called electrophiles. Breaking the double bond ( $ \pi $ -bond) of alkenes, these electrophiles when added to the alkenes produces vicinal dihalides (halogens like $ B{r_2},C{l_2} $ in molecular form). $ B{r_2},C{l_2} $ are the best choice of halogens for halogenation of alkenes.
$ - C = C - + B{r_2}\xrightarrow{{CC{l_4}}} $
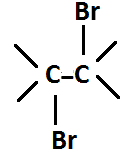
Alkene Bromine 1,2-vicinal dibromide
When $ B{r_2}/CC{l_4} $ is added into the alkenes, bromonium ion and bromide ion are produced with partial positive and partial negative charge respectively. These induced dipoles are formed due to $ \pi - {e^ - } $ cloud of alkene. This bromonium ion attacks the alkene and forms a bond with one of the atoms which leads to formation of a highly unstable intermediate. However, the bromide ion then attacks the intermediate from the opposite side and forms1,2-dibromide (trans- dibromo products) that are highly stable.
Since the attack is from the opposite side, so the anti addition is followed.
So, the given statement is false and the value entered is 0.
Note:
Fluorine reacts vigorously and forms many side products whereas addition of iodine is thermodynamically not supported. When $ B{r_2} $ reacts with $ CC{l_4} $ , dipole induced dipole interactions between the molecules occur that lead to formation of $ B{r^ + } $ and $ B{r^ - } $ ions. This helps in electrophilic addition reactions in alkenes.
Complete step by step answer:
Elements like chlorine and bromine are $ {e^ - } $ loving species. They are called electrophiles. Breaking the double bond ( $ \pi $ -bond) of alkenes, these electrophiles when added to the alkenes produces vicinal dihalides (halogens like $ B{r_2},C{l_2} $ in molecular form). $ B{r_2},C{l_2} $ are the best choice of halogens for halogenation of alkenes.
$ - C = C - + B{r_2}\xrightarrow{{CC{l_4}}} $

Alkene Bromine 1,2-vicinal dibromide
When $ B{r_2}/CC{l_4} $ is added into the alkenes, bromonium ion and bromide ion are produced with partial positive and partial negative charge respectively. These induced dipoles are formed due to $ \pi - {e^ - } $ cloud of alkene. This bromonium ion attacks the alkene and forms a bond with one of the atoms which leads to formation of a highly unstable intermediate. However, the bromide ion then attacks the intermediate from the opposite side and forms1,2-dibromide (trans- dibromo products) that are highly stable.
Since the attack is from the opposite side, so the anti addition is followed.
So, the given statement is false and the value entered is 0.
Note:
Fluorine reacts vigorously and forms many side products whereas addition of iodine is thermodynamically not supported. When $ B{r_2} $ reacts with $ CC{l_4} $ , dipole induced dipole interactions between the molecules occur that lead to formation of $ B{r^ + } $ and $ B{r^ - } $ ions. This helps in electrophilic addition reactions in alkenes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




