
${H_3}P{O_2}$ and ${H_4}{P_2}{O_7}$ are respectively:
A. Tribasic and tetrabasic acids
B. Dibasic and tetrabasic acids
C. Monobasic and tetrabasic acids
D. Tribasic and dibasic acids
Answer
578.7k+ views
Hint: We know that basicity of an acid is given by the number of ionizable ${H^ + }$ that it can give up.
Complete step by step answer:
We have different theories that can be used to define acids including Arrhenius theory and Brönsted-Lowry theory. According to both of these theories, acids can be defined as ${H^ + }$ donors. For example, hydrochloric acid $\left( {HCl} \right)$ and sulfuric acid$\left( {{H_2}S{O_4}} \right)$ as we can see from their ionization:
$\begin{array}{c}
HCl \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over
{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} {H^ + } + C{l^ - }\\
{H_2}S{O_4} \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over
{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} 2{H^ + } + SO_4^{2 - }
\end{array}$
As we can see that hydrochloric acid has one ionizable ${H^ + }$ whereas sulfuric acid has two ionizable ${H^ + }$. This can form a basis to categorize acids as well and giving a new term basicity of acids which is equal to the number of ionizable ${H^ + }$ present in the acid. Depending on the basicity, we can have monobasic, dibasic, tribasic, tetrabsic acids having $1,2,3\;and\;4$ ionizable ${H^ + }$ respectively and so on.
So, in the above examples, $HCl$ is a monobasic acid whereas ${H_2}S{O_4}$ is a dibasic acid.
Now, let’s have a look at the given acids: ${H_3}P{O_2}$ and ${H_4}{P_2}{O_7}$
The structure of these acids can be shown as follows:
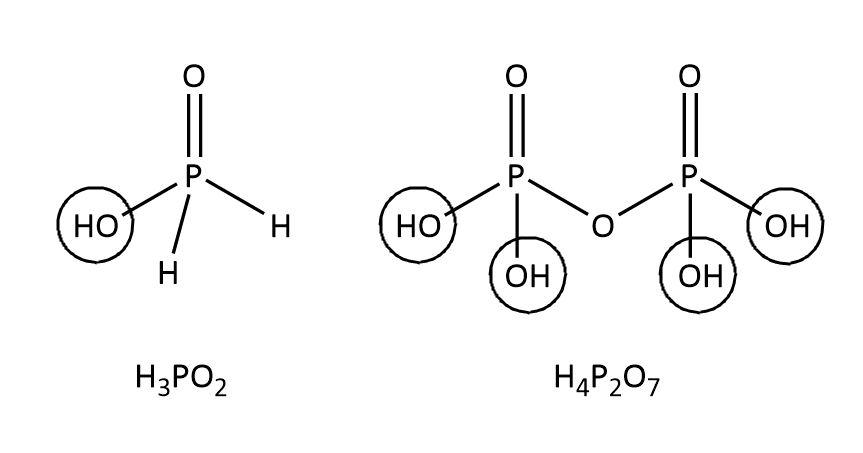
As we can see that in the hypophosphorous acid with the formula ${H_3}P{O_2}$, we have only one $P - OH$ bond whereas other two hydrogens are directly attached to $P$ and are non-ionizable. So, ${H_3}P{O_2}$ is a monobasic acid.
Now, in case of pyrophosphoric acid with the formula ${H_4}{P_2}{O_7}$, we have four $P - OH$ bonds which gives us four ionizable protons. So, ${H_4}{P_2}{O_7}$ is a tetrabasic acid.
Therefore, from the above explanation the correct option is (C).
Note:
We have to look carefully at the structure of as basicity cannot be simply deduced from looking at the chemical formula only.
Complete step by step answer:
We have different theories that can be used to define acids including Arrhenius theory and Brönsted-Lowry theory. According to both of these theories, acids can be defined as ${H^ + }$ donors. For example, hydrochloric acid $\left( {HCl} \right)$ and sulfuric acid$\left( {{H_2}S{O_4}} \right)$ as we can see from their ionization:
$\begin{array}{c}
HCl \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over
{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} {H^ + } + C{l^ - }\\
{H_2}S{O_4} \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over
{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} 2{H^ + } + SO_4^{2 - }
\end{array}$
As we can see that hydrochloric acid has one ionizable ${H^ + }$ whereas sulfuric acid has two ionizable ${H^ + }$. This can form a basis to categorize acids as well and giving a new term basicity of acids which is equal to the number of ionizable ${H^ + }$ present in the acid. Depending on the basicity, we can have monobasic, dibasic, tribasic, tetrabsic acids having $1,2,3\;and\;4$ ionizable ${H^ + }$ respectively and so on.
So, in the above examples, $HCl$ is a monobasic acid whereas ${H_2}S{O_4}$ is a dibasic acid.
Now, let’s have a look at the given acids: ${H_3}P{O_2}$ and ${H_4}{P_2}{O_7}$
The structure of these acids can be shown as follows:

As we can see that in the hypophosphorous acid with the formula ${H_3}P{O_2}$, we have only one $P - OH$ bond whereas other two hydrogens are directly attached to $P$ and are non-ionizable. So, ${H_3}P{O_2}$ is a monobasic acid.
Now, in case of pyrophosphoric acid with the formula ${H_4}{P_2}{O_7}$, we have four $P - OH$ bonds which gives us four ionizable protons. So, ${H_4}{P_2}{O_7}$ is a tetrabasic acid.
Therefore, from the above explanation the correct option is (C).
Note:
We have to look carefully at the structure of as basicity cannot be simply deduced from looking at the chemical formula only.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




