
$ {H_2}{S_2}{O_8} $ and $ {H_2}S{O_5} $ both have $ + 6 $ oxidation state of Sulphur. It is due to the:
(A) Presence of peroxy group
(B) Presence of superoxo group
(C) Presence of neutral group
(D) Presence of ozone
Answer
547.5k+ views
Hint: If we know the structure of $ {H_2}{S_2}{O_8} $ and $ {H_2}S{O_5} $ we can see and check which of the following group is present in these compounds. Also, peroxy group is $ {O_2}^{2 - } $ , superoxo group is $ {O_2}^ - $ , neutral can be $ {O_2} $ and ozone is $ {O_3} $ .
Complete Step by step answer:
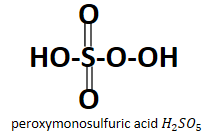
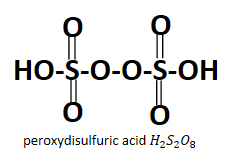
Looking at the structure it is obvious that both the acids have peroxo group attached to them which is $ {O_2}^{2 - } $ , in case of peroxydisulfuric acid it is present in between the two sulfur atoms whereas in case of peroxymonosulfuric acid it is the $ - O - OH $ peroxy group which is linked to the sulfur atom. Also it is very obvious from their names that they have a peroxy group contained in them.
So option (A) is correct.
Additional information:
The oxidation state of the sulfur atom could be calculated assuming all the bonds as ionic and when the bond between sulfur and oxygen is broken then the more electronegative atom which is here is oxygen will carry one negative charge so if again the double bond will be broken then we will have $ {O^{2 - }} $ left and there will be $ + 2 $ charge on sulfur atom. And hence it can be confirmed that sulfur will carry $ + 6 $ charge in both the compounds. Also it is not always the case but is valid for sulfur and phosphorus compounds.
Note:
The difference between the peroxo, superoxo, oxo and ozone should be carefully noted as they have a very slight difference between them and might be confusing sometime. Also while finding the oxidation state you must know the structure of the compound.
Complete Step by step answer:
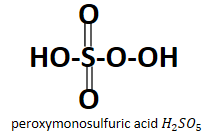

Looking at the structure it is obvious that both the acids have peroxo group attached to them which is $ {O_2}^{2 - } $ , in case of peroxydisulfuric acid it is present in between the two sulfur atoms whereas in case of peroxymonosulfuric acid it is the $ - O - OH $ peroxy group which is linked to the sulfur atom. Also it is very obvious from their names that they have a peroxy group contained in them.
So option (A) is correct.
Additional information:
The oxidation state of the sulfur atom could be calculated assuming all the bonds as ionic and when the bond between sulfur and oxygen is broken then the more electronegative atom which is here is oxygen will carry one negative charge so if again the double bond will be broken then we will have $ {O^{2 - }} $ left and there will be $ + 2 $ charge on sulfur atom. And hence it can be confirmed that sulfur will carry $ + 6 $ charge in both the compounds. Also it is not always the case but is valid for sulfur and phosphorus compounds.
Note:
The difference between the peroxo, superoxo, oxo and ozone should be carefully noted as they have a very slight difference between them and might be confusing sometime. Also while finding the oxidation state you must know the structure of the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




