
What is the ground-state term symbol for the aluminium atom in a magnetic field?
Answer
498.3k+ views
Hint: The term symbol in quantum mechanics is an abbreviated description of the angular momentum quantum numbers in a multi-electron system. Every energy level is not only described by its configuration but also its term symbol. The term symbol usually assumes LS coupling.
Complete Step By Step Answer:
The term symbol has a form of: $ ^{2S + 1}{L_J} $
Where $ 2S + 1 $ is the spin multiplicity, L is the orbital quantum number having values S, P, D, F, G, etc. and J is the total angular momentum quantum number. The value of J ranges from $ {J_{\max }} - {J_{\min }} $ (max to min) . The value of $ {J_{\max }} = |L + S| $ and $ {J_{\min }} = |L - S| $
The spin multiplicity or the total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $ for I no. of electrons. And total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $ for I no. of electrons. If the value of L =0,1,2,3,4, etc. it corresponds to L = S,P,D,F,G, etc, respectively.
We are given the atom Aluminum. The electronic configuration of Aluminum is given as: $ Al:[Ne]3{s^2}3{p^1} $
Diagrammatically it is given as:
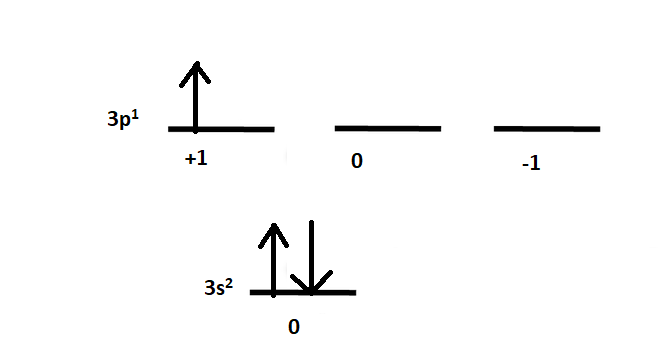
The 3s orbital has two paired electrons and 3p has one unpaired electron. Let us find the term symbols for each orbital one by one.
TERM SYMBOL FOR 3S ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} - \dfrac{1}{2} = 0 $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2(0) + 1 = 1 $ . Spin multiplicity = 1 indicates Singlet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The doubly occupied 3s orbital will have a $ {m_l} = 0 $ . The total angular momentum quantum number L will be: $ L = |0| = 0 \to S $
The term symbol until now can be written as $ ^1S $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |0 - 0| = 0 $ to $ {J_{\max }} = |0 - 0| = 0 $ . Therefore, the value of J will be $ J = 0 $ . The term symbol for 3s orbital will be $ ^1{S_0} $
TERM SYMBOL FOR 3p ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} = \dfrac{1}{2} $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2\left( {\dfrac{1}{2}} \right) + 1 = 2 $ . Spin multiplicity = 2 indicates Doublet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The singly occupied orbital will have a $ {m_l} = + 1 $ . The total angular momentum quantum number L will be: $ L = | + 1| = 1 \to P $
The term symbol until now can be written as $ ^2P $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |1 - \dfrac{1}{2}| = \dfrac{1}{2} $ to $ {J_{\max }} = |1 + \dfrac{1}{2}| = \dfrac{3}{2} $ . Therefore, the value of J will be $ J = \dfrac{1}{2},\dfrac{3}{2} $
The term symbols for 3p orbitals will thus will have two values: $ ^2{P_{\dfrac{1}{2}}}{,^2}{P_{\dfrac{3}{2}}} $
We are asked to find the Ground state term symbol, according to Hund’s rule:
- The term with the largest S is more stable, unless all have the same value of S.
- For terms having the same value of S and L, the subshell that has less than half filled electrons will have the smallest J and vice versa. If it has exactly half-filled electrons J will be 0.
In the given configuration the values of S and L are same, and 3p is less than half filled orbital, therefore 1/2 is more stable than 3/2. The final ground state term symbol is $ ^2{P_{1/2}} $ . This is the required answer.
Note:
If we are asked the ground state term symbol, the value of J will be $ {J_{\min }} = |L - S| $ for less than half filled orbitals and $ {J_{\max }} = |L + S| $ for more than half filled orbitals. In this case the orbital is less than half filled, hence the value of J will be $ {J_{\min }} = |5 - 1| = 4 $ and the ground state term symbol will be $ ^3{H_4} $
Complete Step By Step Answer:
The term symbol has a form of: $ ^{2S + 1}{L_J} $
Where $ 2S + 1 $ is the spin multiplicity, L is the orbital quantum number having values S, P, D, F, G, etc. and J is the total angular momentum quantum number. The value of J ranges from $ {J_{\max }} - {J_{\min }} $ (max to min) . The value of $ {J_{\max }} = |L + S| $ and $ {J_{\min }} = |L - S| $
The spin multiplicity or the total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $ for I no. of electrons. And total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $ for I no. of electrons. If the value of L =0,1,2,3,4, etc. it corresponds to L = S,P,D,F,G, etc, respectively.
We are given the atom Aluminum. The electronic configuration of Aluminum is given as: $ Al:[Ne]3{s^2}3{p^1} $
Diagrammatically it is given as:

The 3s orbital has two paired electrons and 3p has one unpaired electron. Let us find the term symbols for each orbital one by one.
TERM SYMBOL FOR 3S ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} - \dfrac{1}{2} = 0 $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2(0) + 1 = 1 $ . Spin multiplicity = 1 indicates Singlet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The doubly occupied 3s orbital will have a $ {m_l} = 0 $ . The total angular momentum quantum number L will be: $ L = |0| = 0 \to S $
The term symbol until now can be written as $ ^1S $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |0 - 0| = 0 $ to $ {J_{\max }} = |0 - 0| = 0 $ . Therefore, the value of J will be $ J = 0 $ . The term symbol for 3s orbital will be $ ^1{S_0} $
TERM SYMBOL FOR 3p ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} = \dfrac{1}{2} $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2\left( {\dfrac{1}{2}} \right) + 1 = 2 $ . Spin multiplicity = 2 indicates Doublet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The singly occupied orbital will have a $ {m_l} = + 1 $ . The total angular momentum quantum number L will be: $ L = | + 1| = 1 \to P $
The term symbol until now can be written as $ ^2P $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |1 - \dfrac{1}{2}| = \dfrac{1}{2} $ to $ {J_{\max }} = |1 + \dfrac{1}{2}| = \dfrac{3}{2} $ . Therefore, the value of J will be $ J = \dfrac{1}{2},\dfrac{3}{2} $
The term symbols for 3p orbitals will thus will have two values: $ ^2{P_{\dfrac{1}{2}}}{,^2}{P_{\dfrac{3}{2}}} $
We are asked to find the Ground state term symbol, according to Hund’s rule:
- The term with the largest S is more stable, unless all have the same value of S.
- For terms having the same value of S and L, the subshell that has less than half filled electrons will have the smallest J and vice versa. If it has exactly half-filled electrons J will be 0.
In the given configuration the values of S and L are same, and 3p is less than half filled orbital, therefore 1/2 is more stable than 3/2. The final ground state term symbol is $ ^2{P_{1/2}} $ . This is the required answer.
Note:
If we are asked the ground state term symbol, the value of J will be $ {J_{\min }} = |L - S| $ for less than half filled orbitals and $ {J_{\max }} = |L + S| $ for more than half filled orbitals. In this case the orbital is less than half filled, hence the value of J will be $ {J_{\min }} = |5 - 1| = 4 $ and the ground state term symbol will be $ ^3{H_4} $
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




