
When Grignard reagents react with ethanol, which product is formed?
Answer
498k+ views
Hint: Grignard reagent is used in many chemical reactions and it is represented as $ RMgX $ . Where $ R $ represents the attached alkyl group, $ Mg $ represents the magnesium metal and $ X $ represents the halide. Therefore, it is commonly known as magnesium halide.
Complete answer:
Grignard reagent acts as a very good nucleophile therefore it has a tendency to attack the electrophiles to form the compound. The electrophile which combines with Grignard reagent may be an epoxide, or any carbonyl center.
When Grignard reagents react with ethanol, due to nucleophilic nature it tends to extract the hydrogen atom attached with the hydroxyl group.
The reaction between the ethanol and Grignard reagent is considered as an acid base reaction because alcohol acts as an acid while Grignard reagent acts as a base.
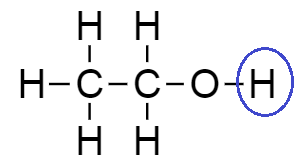
From the structure of ethanol, we see that there is one hydrogen which is attached directly with an oxygen atom hence, it is considered as an acidic hydrogen.
Hence when Grignard reagent with acidic hydrogen process of neutralization occurs by removal of acidic hydrogen from alcohol.
The alkyl group of the Grignard reagent combines with hydrogen extracted from alcohol to form an alkane.
For example- reaction between ethanol $ {C_2}{H_5}OH $ and methyl magnesium chloride $ C{H_3}MgCl $ .
$ {C_2}{H_5}OH $ $ + $ $ C{H_3}MgCl $ $ \to C{H_4} + {C_2}{H_5}OMgCl $
$ \Rightarrow $ Grignard reagent reacts with ethanol to produce alkane.
Note:
Always remember that the alkyl group of Grignard reagent is used in the newly formed alkane. Grignard reagent is also used in the synthesis of alcohol by reacting with carbonyl group aldehyde or ketone. Grignard reagent is used to prepare aliphatic as well as aromatic alcohol.
Complete answer:
Grignard reagent acts as a very good nucleophile therefore it has a tendency to attack the electrophiles to form the compound. The electrophile which combines with Grignard reagent may be an epoxide, or any carbonyl center.
When Grignard reagents react with ethanol, due to nucleophilic nature it tends to extract the hydrogen atom attached with the hydroxyl group.
The reaction between the ethanol and Grignard reagent is considered as an acid base reaction because alcohol acts as an acid while Grignard reagent acts as a base.

From the structure of ethanol, we see that there is one hydrogen which is attached directly with an oxygen atom hence, it is considered as an acidic hydrogen.
Hence when Grignard reagent with acidic hydrogen process of neutralization occurs by removal of acidic hydrogen from alcohol.
The alkyl group of the Grignard reagent combines with hydrogen extracted from alcohol to form an alkane.
For example- reaction between ethanol $ {C_2}{H_5}OH $ and methyl magnesium chloride $ C{H_3}MgCl $ .
$ {C_2}{H_5}OH $ $ + $ $ C{H_3}MgCl $ $ \to C{H_4} + {C_2}{H_5}OMgCl $
$ \Rightarrow $ Grignard reagent reacts with ethanol to produce alkane.
Note:
Always remember that the alkyl group of Grignard reagent is used in the newly formed alkane. Grignard reagent is also used in the synthesis of alcohol by reacting with carbonyl group aldehyde or ketone. Grignard reagent is used to prepare aliphatic as well as aromatic alcohol.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




