
How do Grignard reagent react with an acetone?
Answer
541.2k+ views
Hint :We know that the Grignard reagent is organometallic reagent comprising of alkyl, allyl, vinyl or aryl-Magnesium halides which undergoes reaction with carbonyl group in an aldehyde or ketone, this reaction is known as Grignard reaction. It has been a historically useful reaction for generating carbon-carbon single bonds. The Grignard reagent is obtained by reaction of an organic halide with Magnesium metal.
Complete Step By Step Answer:
The reaction between Grignard reagent and ketone forms a tertiary alcohol as the product of the reaction, The reaction proceeds via one single step and involves the formation of a six membered transition state which is formed when the attack of Grignard reagent occurs upon the Carbonyl center of ketone after this we generally see a protonation step which completes the reaction.
The complete mechanism is shown below:
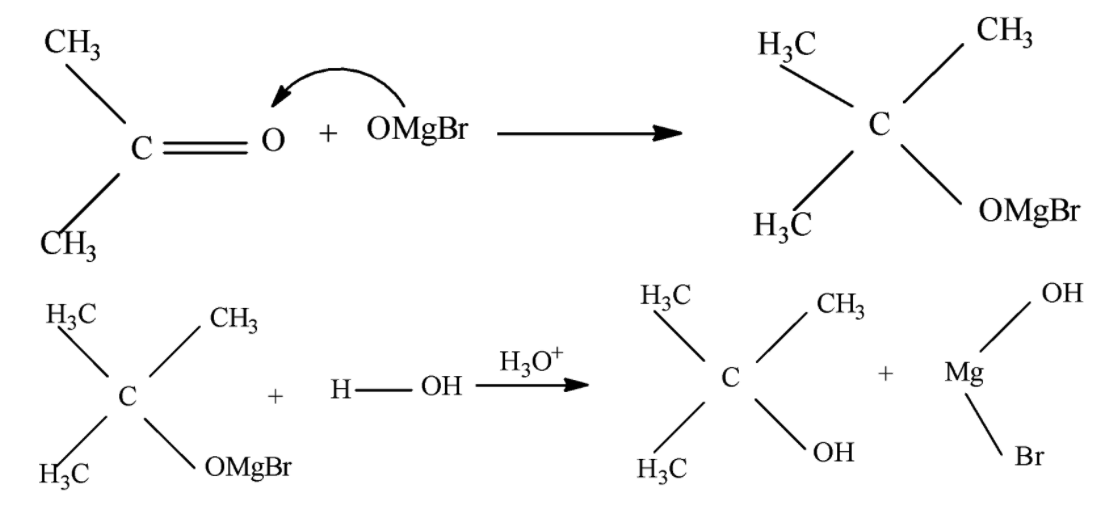
During the course of reaction the carbonyl species acts as the nucleophile and the Grignard reagent acts as the Electrophile. Sometimes when the ketone is unsymmetrical and the alkyl part of Grignard reagent is also unsymmetrical we obtain a chiral alcohol as the final product whose configuration depends on the nature of the side of approach.
Note :
Note that when Grignard reagent is used we must be careful to avoid using water as a solvent as Grignard reagent is highly prone to react with water, also in case of Alpha beta unsaturated ketones Grignard reagent always attacks the carbonyl center preferably due to favored hard-hard interaction at that center.
Complete Step By Step Answer:
The reaction between Grignard reagent and ketone forms a tertiary alcohol as the product of the reaction, The reaction proceeds via one single step and involves the formation of a six membered transition state which is formed when the attack of Grignard reagent occurs upon the Carbonyl center of ketone after this we generally see a protonation step which completes the reaction.
The complete mechanism is shown below:

During the course of reaction the carbonyl species acts as the nucleophile and the Grignard reagent acts as the Electrophile. Sometimes when the ketone is unsymmetrical and the alkyl part of Grignard reagent is also unsymmetrical we obtain a chiral alcohol as the final product whose configuration depends on the nature of the side of approach.
Note :
Note that when Grignard reagent is used we must be careful to avoid using water as a solvent as Grignard reagent is highly prone to react with water, also in case of Alpha beta unsaturated ketones Grignard reagent always attacks the carbonyl center preferably due to favored hard-hard interaction at that center.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




