
Graphite is used as a lubricant due to:
A. The slippery nature
B. The giant structure
C. High refractive index
D. High IP value of carbon
Answer
590.7k+ views
Hint: Recall that graphite is an allotrope of carbon. Think about the structure of graphite and how the carbon atoms are bonded. This will give you an idea about the property of lubrication.
Complete step by step solution:
Graphite is an allotropic form of carbon that is found in sheets. One carbon is attached to three other carbon atoms and form consecutive hexagons that look like a beehive. These hexagons are linear in nature and form sheets of such hexagonal carbon. These sheets are then stacked on top of each other and have a very weak bonding force between them. This allows them to slip over each other. The length of one side of the hexagon formed is 142pm and the distance between 2 sheets is measured to be 335m. This property that allows graphite layers to slip over each other facilitates the ability of graphite to act as a lubricant. The structure of graphite is as follows:
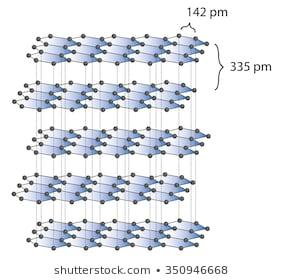
Since the valency of carbon is 4 and it bonds with only 3 other atoms in this structure, there are a lot of free available electrons. These electrons are then used to conduct electricity through the graphite. His makes graphite a good conductor of electricity.
Hence, the answer to this question is ‘A. Its slippery nature’
Note: Although the structure of graphite is quite large, it is not the factor that allows it to be a good lubricant. But, may cause it to be a hindrance. The refractive index of graphite is high, but this does not contribute to the fact that graphite is used as a lubricant. The IP value refers to the ionization potential of the substance. The IP value of graphite is high since it has many free electrons but it allows graphite to be a good conductor of electricity and not a good lubricant.
Complete step by step solution:
Graphite is an allotropic form of carbon that is found in sheets. One carbon is attached to three other carbon atoms and form consecutive hexagons that look like a beehive. These hexagons are linear in nature and form sheets of such hexagonal carbon. These sheets are then stacked on top of each other and have a very weak bonding force between them. This allows them to slip over each other. The length of one side of the hexagon formed is 142pm and the distance between 2 sheets is measured to be 335m. This property that allows graphite layers to slip over each other facilitates the ability of graphite to act as a lubricant. The structure of graphite is as follows:

Since the valency of carbon is 4 and it bonds with only 3 other atoms in this structure, there are a lot of free available electrons. These electrons are then used to conduct electricity through the graphite. His makes graphite a good conductor of electricity.
Hence, the answer to this question is ‘A. Its slippery nature’
Note: Although the structure of graphite is quite large, it is not the factor that allows it to be a good lubricant. But, may cause it to be a hindrance. The refractive index of graphite is high, but this does not contribute to the fact that graphite is used as a lubricant. The IP value refers to the ionization potential of the substance. The IP value of graphite is high since it has many free electrons but it allows graphite to be a good conductor of electricity and not a good lubricant.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




