
When glycerol is treated with excess of $HI$, it produces:
A. \[2 - iodopropane\]
B. \[allyl{\text{ }}iodide\]
C. \[propene\]
D. \[glycerol{\text{ }}triiodide\]
Answer
594.3k+ views
Hint: Glycerol is also called as Glycerin and is a colorless and an odorless liquid, and is non-toxic in nature and has a sweet taste, its IUPAC name is \[propane - 1,2,3 - triol\]. It has many antiviral and antifungal properties so it is very important for treating wounds and burns.
Complete answer:
Glycerol is also referred to as polyol as it consists of many alcohols. When we talk about its solubility it is soluble in water and is hygroscopic in nature which means it has a tendency to attract the water molecules around it.
Now, when glycerol is treated with an excess of \[HI\], that reactions follow in steps as follows:
In the first step, a molecule of glycerol reacts with three moles of \[HI\;\]molecules to give a product\[1,2,3 - triiodopropane\], which is unstable in nature and this formed intermediate loses a molecule of iodine and hence the formation of allyl iodide takes place, then again the formed Allyl iodide adds a molecule of \[HI\] to obtain an unstable molecule which loses a molecule of iodine to form propene. Then the formed propene undergoes an addition reaction and \[HI\] molecule is added to the propene to give \[2 - iodopropane\]. This can be explained by the reaction as follows:
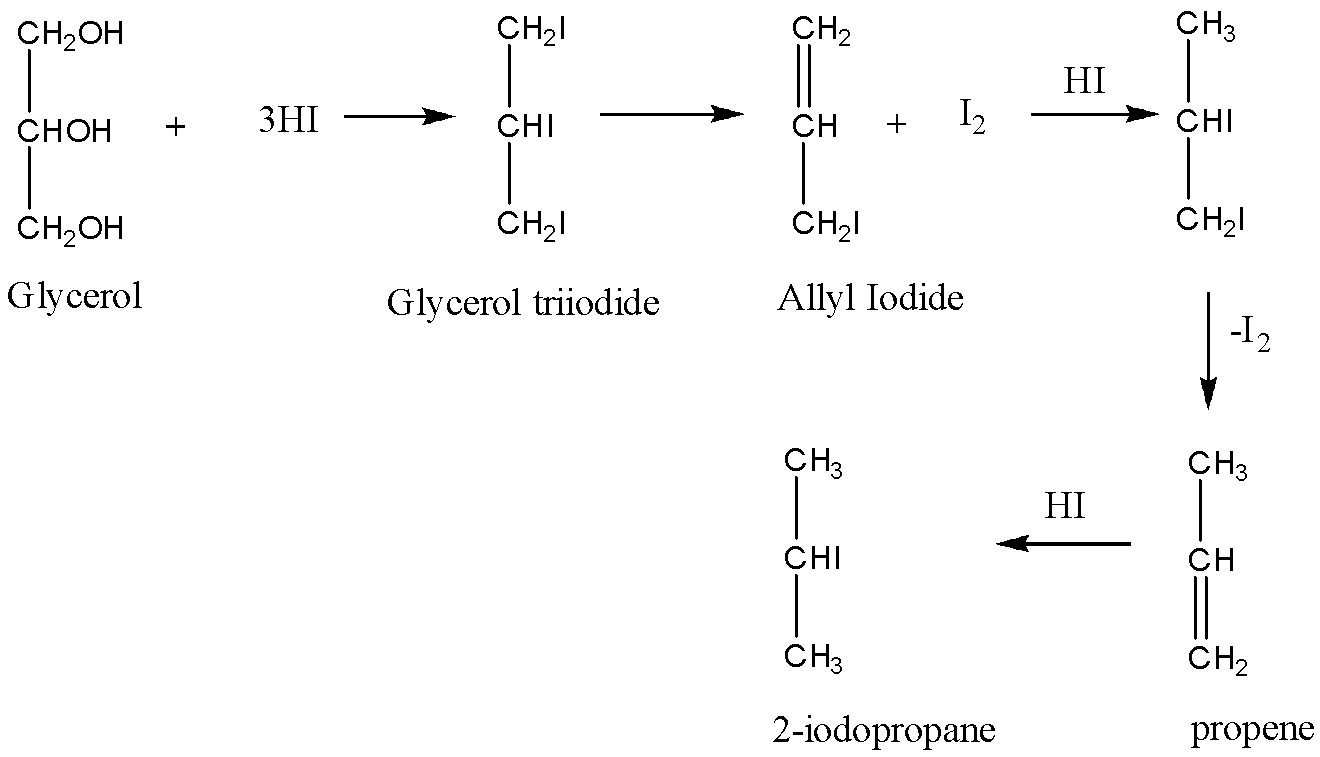
So, the correct answer is \[2 - iodopropane\], Option A.
Note:
In such type of questions, one must answer the final product which is formed, well in this question all of the given options are formed in the reaction either as an intermediate or as a final product, in the first step the glycerol triiodide is formed followed by the formation of allyl iodide and then followed by propene and finally the \[2 - iodopropane\].
Complete answer:
Glycerol is also referred to as polyol as it consists of many alcohols. When we talk about its solubility it is soluble in water and is hygroscopic in nature which means it has a tendency to attract the water molecules around it.
Now, when glycerol is treated with an excess of \[HI\], that reactions follow in steps as follows:
In the first step, a molecule of glycerol reacts with three moles of \[HI\;\]molecules to give a product\[1,2,3 - triiodopropane\], which is unstable in nature and this formed intermediate loses a molecule of iodine and hence the formation of allyl iodide takes place, then again the formed Allyl iodide adds a molecule of \[HI\] to obtain an unstable molecule which loses a molecule of iodine to form propene. Then the formed propene undergoes an addition reaction and \[HI\] molecule is added to the propene to give \[2 - iodopropane\]. This can be explained by the reaction as follows:

So, the correct answer is \[2 - iodopropane\], Option A.
Note:
In such type of questions, one must answer the final product which is formed, well in this question all of the given options are formed in the reaction either as an intermediate or as a final product, in the first step the glycerol triiodide is formed followed by the formation of allyl iodide and then followed by propene and finally the \[2 - iodopropane\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




