
Glucose reacts with excess of with phenylhydrazine and forms:
A. Glucosazone
B. Glucose phenylhydrazone
C. Glucose oxime
D. sorbitol
Answer
567k+ views
Hint:To answer this question we should know the structure of the glucose and the reaction caused by phenylhydrazine. Glucose is a hexose sugar having six carbon atoms and one aldehydic functional group. The chemical formula of phenyl hydrazine is ${\text{NH}} - {\text{N}}{{\text{H}}_{\text{2}}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}$. Phenyl hydrazine causes the removal of the water molecules.
Complete answer:
The reducing sugars react with phenylhydrazine and form a compound that is known as osazones.
By the reaction of phenyl hydrazine, carbonyl group, and alpha-carbon get oxidized.
When glucose reacts with phenylhydrazine, the products glucosazone, ammonia, and water form.
The reaction of glucose with phenylhydrazine is shown as follows:
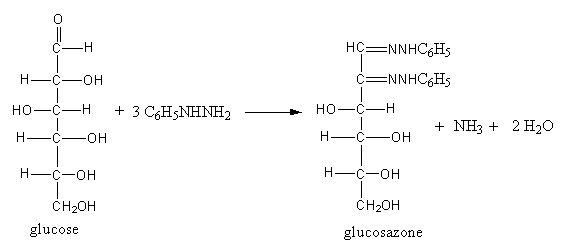
So, glucose reacts with an excess of phenylhydrazine and forms glucosazone.
Therefore, option (A) Glucosazone, is correct.
Note:The reaction of glucose with phenylhydrazine gives glucose phenylhydrazone whereas the reaction of glucose with excess phenylhydrazine gives osazone. The sugar having free aldehyde or ketone groups is known as reducing sugars. All monosaccharides are reducing sugar. The formation of osazone is used for the identification of reducing sugars. Osazone are coloured compounds and osazone of different sugars have different crystal shapes, so they can be identified easily. Glucose and fructose form the same osazone.
Complete answer:
The reducing sugars react with phenylhydrazine and form a compound that is known as osazones.
By the reaction of phenyl hydrazine, carbonyl group, and alpha-carbon get oxidized.
When glucose reacts with phenylhydrazine, the products glucosazone, ammonia, and water form.
The reaction of glucose with phenylhydrazine is shown as follows:

So, glucose reacts with an excess of phenylhydrazine and forms glucosazone.
Therefore, option (A) Glucosazone, is correct.
Note:The reaction of glucose with phenylhydrazine gives glucose phenylhydrazone whereas the reaction of glucose with excess phenylhydrazine gives osazone. The sugar having free aldehyde or ketone groups is known as reducing sugars. All monosaccharides are reducing sugar. The formation of osazone is used for the identification of reducing sugars. Osazone are coloured compounds and osazone of different sugars have different crystal shapes, so they can be identified easily. Glucose and fructose form the same osazone.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




