
Given the structural formula for the following
a) Methanoic acid.
Answer
577.2k+ views
Hint:Identify the number of atoms of each element present in methanoic acid. Identify the functional group present in methanoic acid. Identify how different atoms are connected in methanoic acid.
Complete answer:
We can also call Methanoic acid as formic acid. The common name is formic acid as it is obtained from red ant which is known as ‘formica’ in Latin. Methanoic acid is the IUPAC name or systematic name.
Methanoic acid is a carboxylic acid and contains a carboxylic functional group. The parent hydrocarbon is methane.
Formic acid is a colorless liquid that fumes and has a pungent acidic odor. In industry, it is sometimes used as a protic solvent. Methanoic acid is corrosive in nature. We can use formic acid as a reducing agent to reduce sodium dichromate or potassium dichromate.
One molecule of Methanoic acid contains one carbon atom, two oxygen atoms and two hydrogen atoms. The chemical formula of Methanoic acid is \[{\text{HCOOH}}\] or \[{{\text{H}}_2}{\text{C}}{{\text{O}}_2}\] .
Let us write the structural formula for the methanoic acid as shown below:
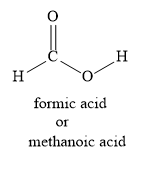
Let us write the Lewis dot structure for methanoic acid as shown below:
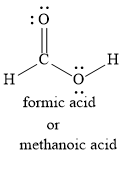
We can also write the Lewis dot structure for methanoic acid as shown below:
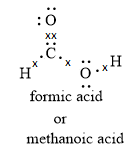
Note:When formic acid loses a proton, it forms a formation ion. The formation ion is resonance stabilized as the negative charge is delocalized over two oxygen atoms.
Complete answer:
We can also call Methanoic acid as formic acid. The common name is formic acid as it is obtained from red ant which is known as ‘formica’ in Latin. Methanoic acid is the IUPAC name or systematic name.
Methanoic acid is a carboxylic acid and contains a carboxylic functional group. The parent hydrocarbon is methane.
Formic acid is a colorless liquid that fumes and has a pungent acidic odor. In industry, it is sometimes used as a protic solvent. Methanoic acid is corrosive in nature. We can use formic acid as a reducing agent to reduce sodium dichromate or potassium dichromate.
One molecule of Methanoic acid contains one carbon atom, two oxygen atoms and two hydrogen atoms. The chemical formula of Methanoic acid is \[{\text{HCOOH}}\] or \[{{\text{H}}_2}{\text{C}}{{\text{O}}_2}\] .
Let us write the structural formula for the methanoic acid as shown below:

Let us write the Lewis dot structure for methanoic acid as shown below:

We can also write the Lewis dot structure for methanoic acid as shown below:
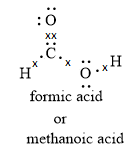
Note:When formic acid loses a proton, it forms a formation ion. The formation ion is resonance stabilized as the negative charge is delocalized over two oxygen atoms.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




