
Give the structure of the following compounds.
4-Nitropropiophenone
Answer
519.6k+ views
Hint :The placement of the atoms, not the electrons, is described by molecular structure. We call the geometry that contains all electron pairs the electron-pair geometry to distinguish between these two circumstances. The molecular structure is the structure that solely comprises the location of the atoms in the molecule.
Complete Step By Step Answer:
Organic compounds with one or more nitro functional groups ($N{O_2}$) are known as nitro compounds. The nitro group is one of the most often utilised explosophores (functional groups that combine to form a compound explosive). In addition, the nitro group is a powerful electron-withdrawing group. CH bonds alpha (adjacent) to the nitro group might be acidic due to this characteristic.
The inclusion of nitro groups in aromatic compounds slows electrophilic aromatic substitution but speeds up nucleophilic aromatic substitution for comparable reasons. Nitrogen groups are uncommon in nature. Nitration processes, which begin with nitric acid, virtually always yield them. A phenone is an aromatic molecule in which the carbonyl keto (O=C) group is joined to the benzene group directly. For example, benzophenone, acetophenone, and others.
The functional component in this chemical is the ketone group, and a nitro group is bonded at the fourth position in relation to the ketone group. As a result, the structure of this chemical is as follows:
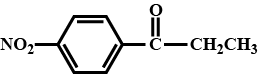
Note :
Although not commonly used, oxo is the IUPAC terminology for the oxo group (=O) and is used as a prefix when the ketone is not the most important component. However, other prefixes are also used. The ketone functional group is used to describe a number of common compounds (mostly in biochemistry).
Complete Step By Step Answer:
Organic compounds with one or more nitro functional groups ($N{O_2}$) are known as nitro compounds. The nitro group is one of the most often utilised explosophores (functional groups that combine to form a compound explosive). In addition, the nitro group is a powerful electron-withdrawing group. CH bonds alpha (adjacent) to the nitro group might be acidic due to this characteristic.
The inclusion of nitro groups in aromatic compounds slows electrophilic aromatic substitution but speeds up nucleophilic aromatic substitution for comparable reasons. Nitrogen groups are uncommon in nature. Nitration processes, which begin with nitric acid, virtually always yield them. A phenone is an aromatic molecule in which the carbonyl keto (O=C) group is joined to the benzene group directly. For example, benzophenone, acetophenone, and others.
The functional component in this chemical is the ketone group, and a nitro group is bonded at the fourth position in relation to the ketone group. As a result, the structure of this chemical is as follows:

Note :
Although not commonly used, oxo is the IUPAC terminology for the oxo group (=O) and is used as a prefix when the ketone is not the most important component. However, other prefixes are also used. The ketone functional group is used to describe a number of common compounds (mostly in biochemistry).
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




