
Give the relationship between the steric hindrance and the rate of esterification?
Answer
520.5k+ views
Hint :To solve this question, first understand both the terms separately and then try to carve out the relationship that binds them. So steric hindrance is the congestion experienced by the atom in the molecule due to the physical presence of other surrounding atoms or molecules.
Complete Step By Step Answer:
So, steric hindrance is generally due to heavy bulky groups surrounding the small molecule or atom. This steric hindrance prevents the atom or molecule from undergoing the desired chemical reaction.
Now, we had an idea about steric hindrance, so now let’s discuss about esterification
Esterification is the process of forming ester bonds by the reaction between an organic acid and the alcohol. In this reaction at least one product has the ester bonds and the water is always released out.
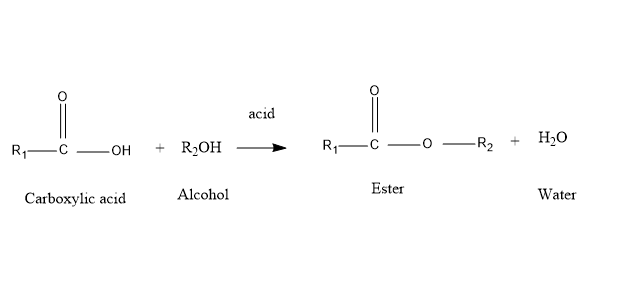
So, by understanding above both the definitions we can say that the steric hindrance blocks the reaction of esterification, it means that steric hindrance slows down the rate of esterification.
Therefore our answer is - that steric hindrance is inversely proportional to the rate of esterification.
The greater the steric hindrance, the lower the rate of esterification.
Note :
Steric hindrance always is not a problematic phenomenon, sometimes it can be a helpful process .Like steric hindrance is often exploited to prevent the side reactions or to prevent the molecule from undergoing unnecessary reactions.
Complete Step By Step Answer:
So, steric hindrance is generally due to heavy bulky groups surrounding the small molecule or atom. This steric hindrance prevents the atom or molecule from undergoing the desired chemical reaction.
Now, we had an idea about steric hindrance, so now let’s discuss about esterification
Esterification is the process of forming ester bonds by the reaction between an organic acid and the alcohol. In this reaction at least one product has the ester bonds and the water is always released out.

So, by understanding above both the definitions we can say that the steric hindrance blocks the reaction of esterification, it means that steric hindrance slows down the rate of esterification.
Therefore our answer is - that steric hindrance is inversely proportional to the rate of esterification.
The greater the steric hindrance, the lower the rate of esterification.
Note :
Steric hindrance always is not a problematic phenomenon, sometimes it can be a helpful process .Like steric hindrance is often exploited to prevent the side reactions or to prevent the molecule from undergoing unnecessary reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




