
Give the major product of the following reaction:
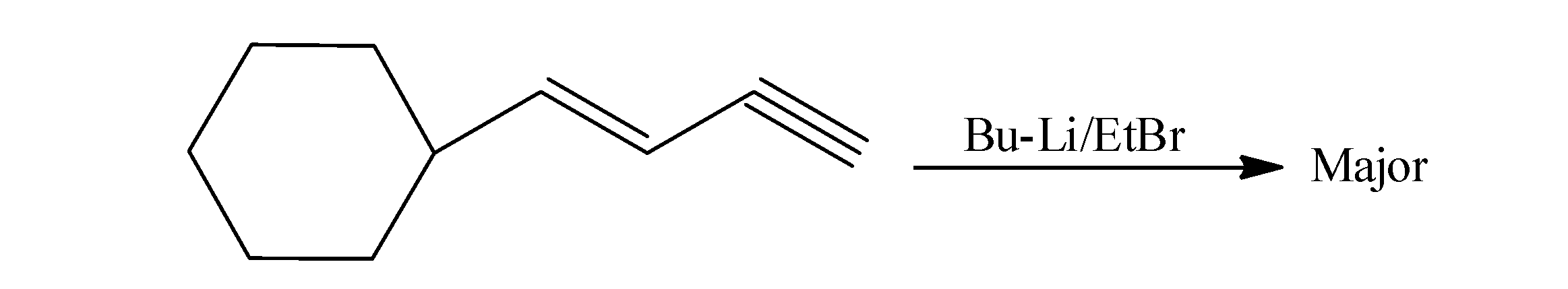
(a)
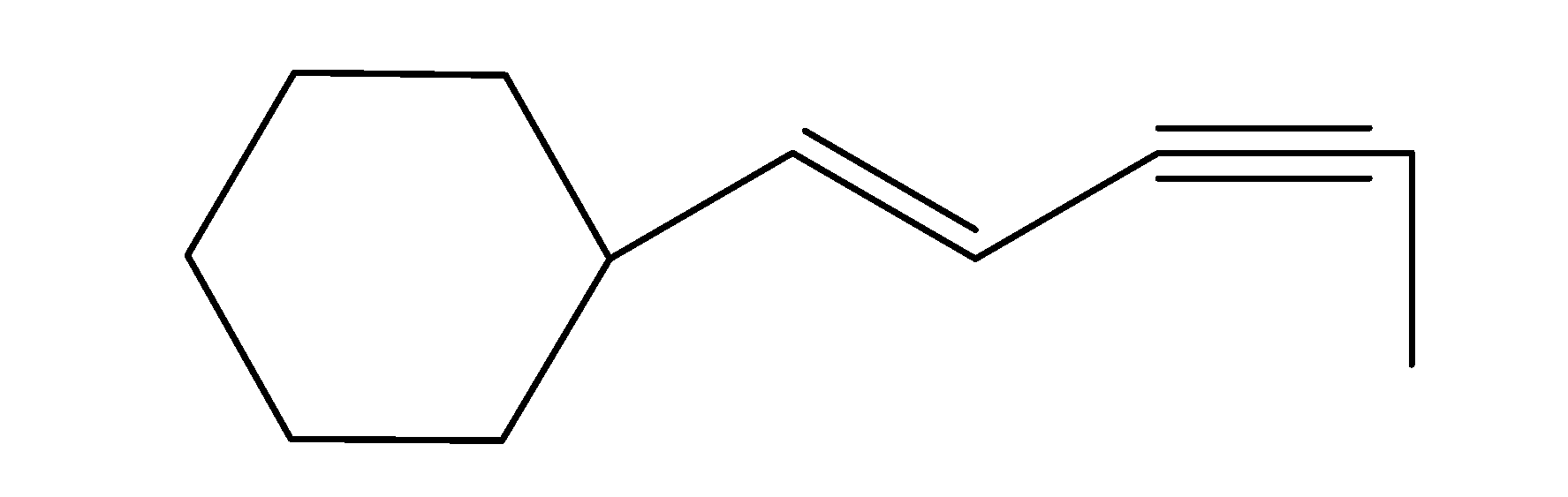
(b)
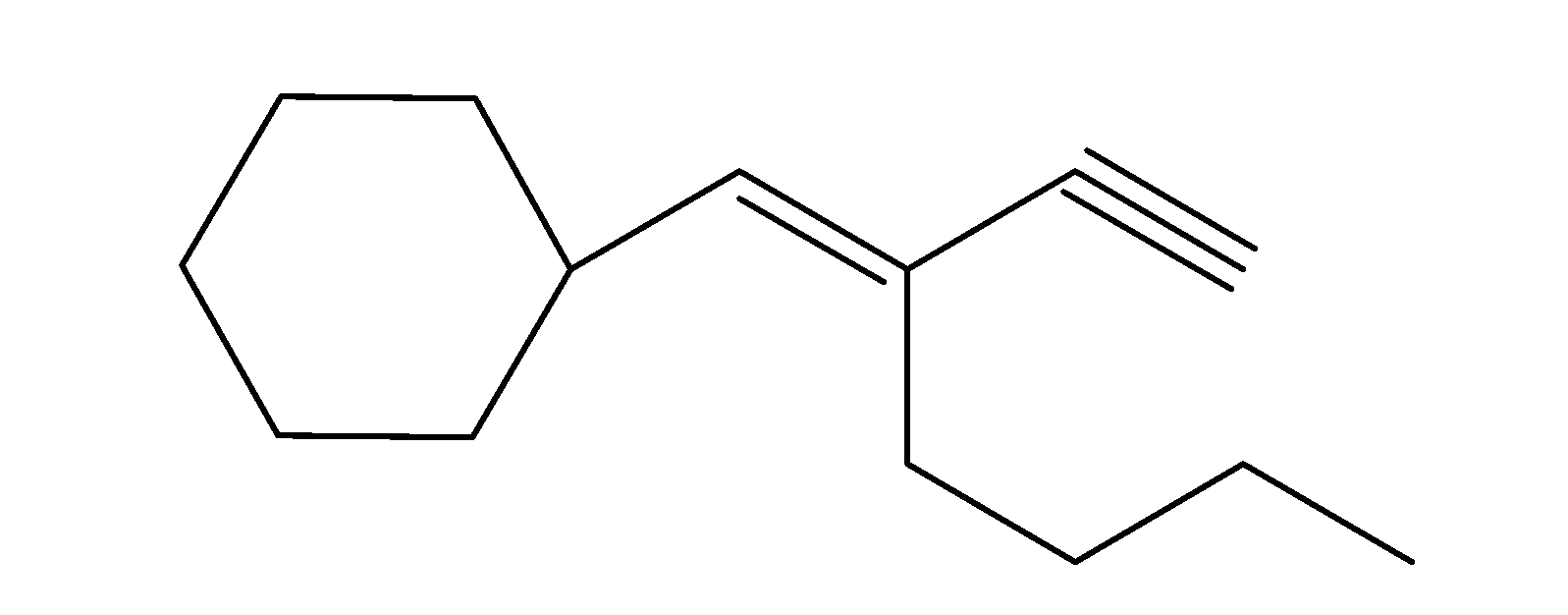
(c)

(d)
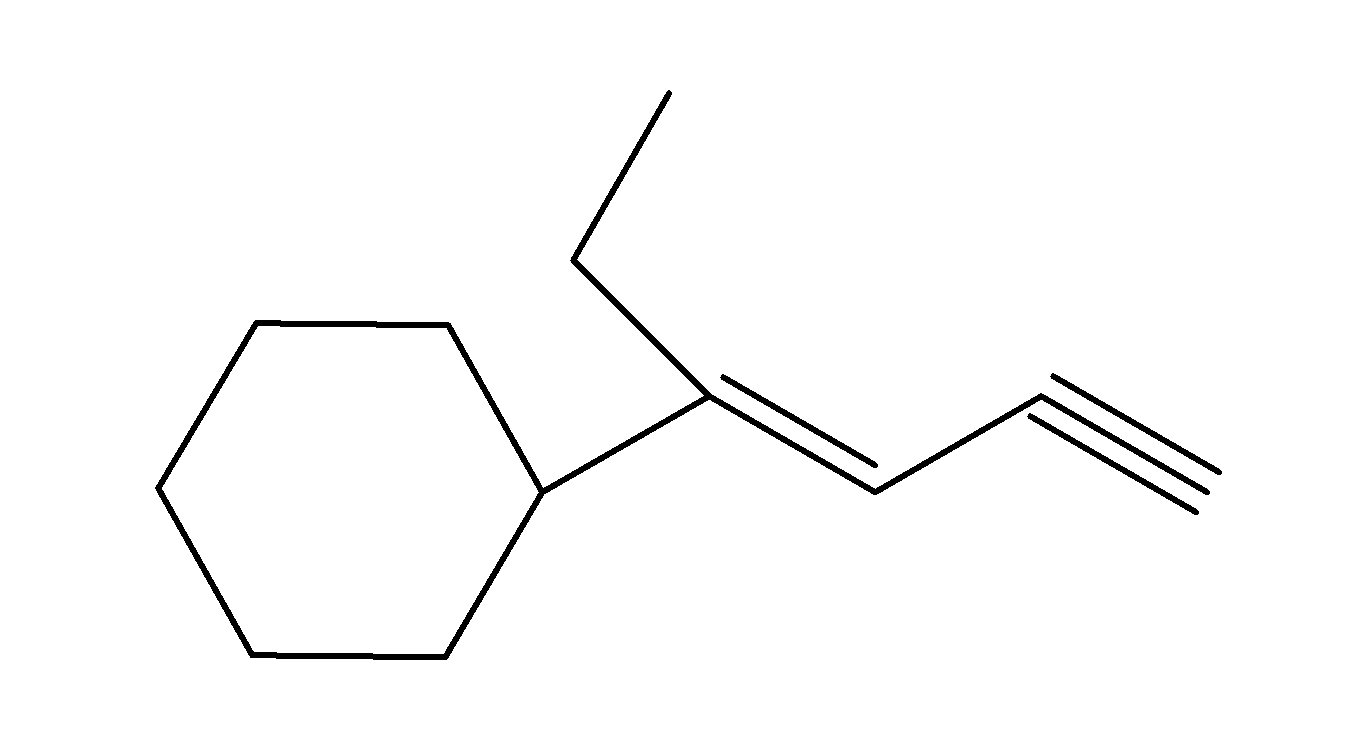





Answer
573.9k+ views
Hint: When an aromatic alkyl unsaturated compound undergoes reaction with the organolithium compound, it results into the reduction i.e. the lithium removes the hydrogen atom and the alkyl group from organolithium compound forms the bond with the aromatic alkyl unsaturated compound and results in the formation of major product. Now identify the product.
Complete Solution :
First of all, let’s discuss about the butyllithium i.e. Bu-Li. The reagent butyl lithium is an organolithium compound.
Now what are organolithium compounds? By organolithium compounds we mean those compounds in which the lithium atom is directly linked to the carbon atom. These compounds are very reactive.
The organolithium compounds can be easily prepared by the reaction of an alkyl halide with lithium or by metal-metal exchange as,
The general reactions are as follows:
From alkyl halide:
$R-X+2Li\xrightarrow{dry\text{ }hexane}R-Li+ LiX $
By metal-metal exchange:
${{R}_{2}}Hg+2Li\xrightarrow{ligroin}2RLi+Hg$
Now considering the statement as:
When an aromatic alkyl compound which consists of both the double and triple undergoes reaction with organolithium compounds ( butyl lithium i.e. Bu-Li), it undergoes reduction and results in the removal of hydrogen which combines with the lithium (Li)of the organolithium compound (Bu-Li).
The alkyl group (Bu) from the organolithium compound (Bu-Li) forms a bond with the aromatic alkyl compound and results in the formation of the major product.
So, the product of the above given reaction with butyllithium is as;
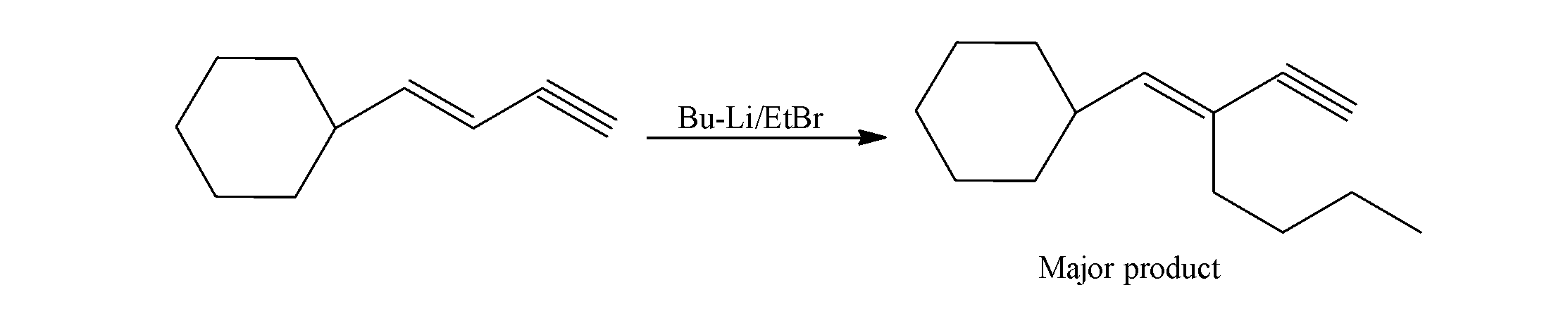
So, the correct answer is “Option B”.
Note: These are even more reactive than the Grignard reagents. It is so because the C-Li bond in the organolithium compounds is more than (electronegativity difference=1.6) C-Mg bond in the Grignard reagents (electronegativity difference = 1.3)
Complete Solution :
First of all, let’s discuss about the butyllithium i.e. Bu-Li. The reagent butyl lithium is an organolithium compound.
Now what are organolithium compounds? By organolithium compounds we mean those compounds in which the lithium atom is directly linked to the carbon atom. These compounds are very reactive.
The organolithium compounds can be easily prepared by the reaction of an alkyl halide with lithium or by metal-metal exchange as,
The general reactions are as follows:
From alkyl halide:
$R-X+2Li\xrightarrow{dry\text{ }hexane}R-Li+ LiX $
By metal-metal exchange:
${{R}_{2}}Hg+2Li\xrightarrow{ligroin}2RLi+Hg$
Now considering the statement as:
When an aromatic alkyl compound which consists of both the double and triple undergoes reaction with organolithium compounds ( butyl lithium i.e. Bu-Li), it undergoes reduction and results in the removal of hydrogen which combines with the lithium (Li)of the organolithium compound (Bu-Li).
The alkyl group (Bu) from the organolithium compound (Bu-Li) forms a bond with the aromatic alkyl compound and results in the formation of the major product.
So, the product of the above given reaction with butyllithium is as;

So, the correct answer is “Option B”.
Note: These are even more reactive than the Grignard reagents. It is so because the C-Li bond in the organolithium compounds is more than (electronegativity difference=1.6) C-Mg bond in the Grignard reagents (electronegativity difference = 1.3)
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




