
Give the IUPAC names of following compounds:
(A)
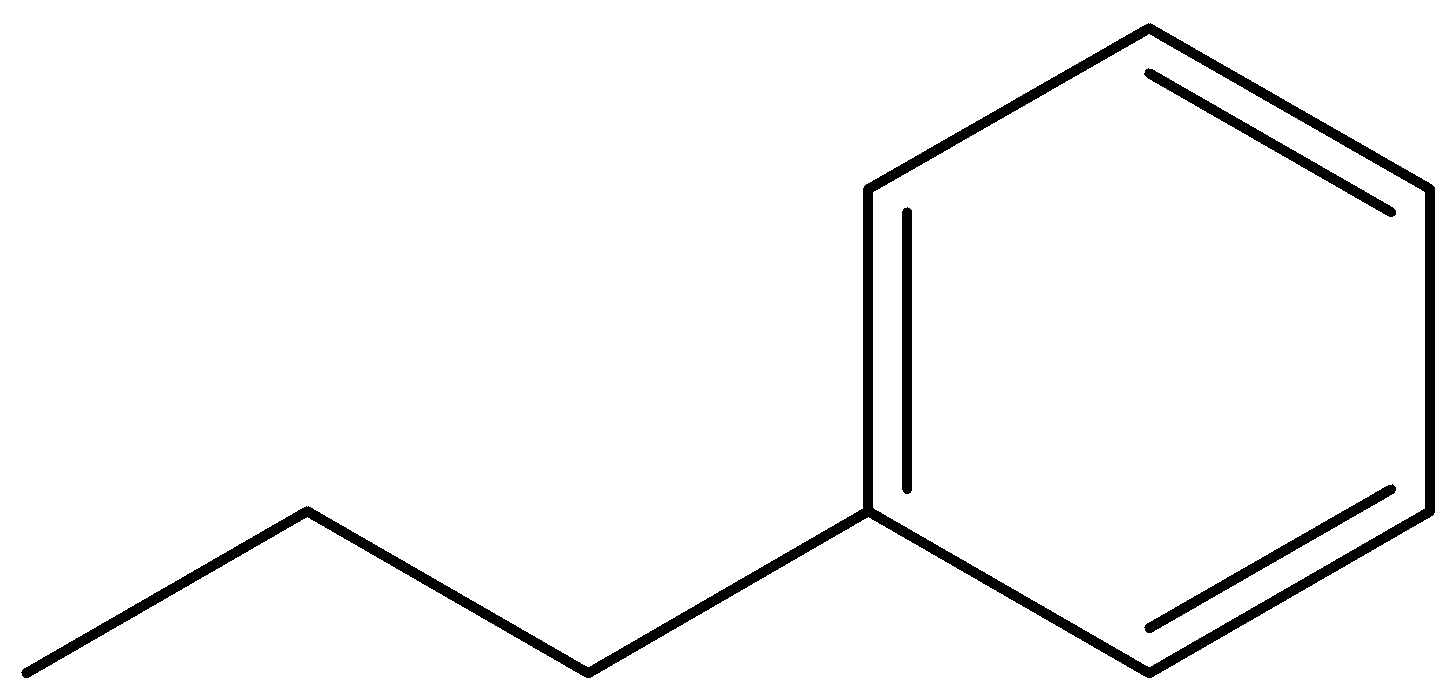
(B)
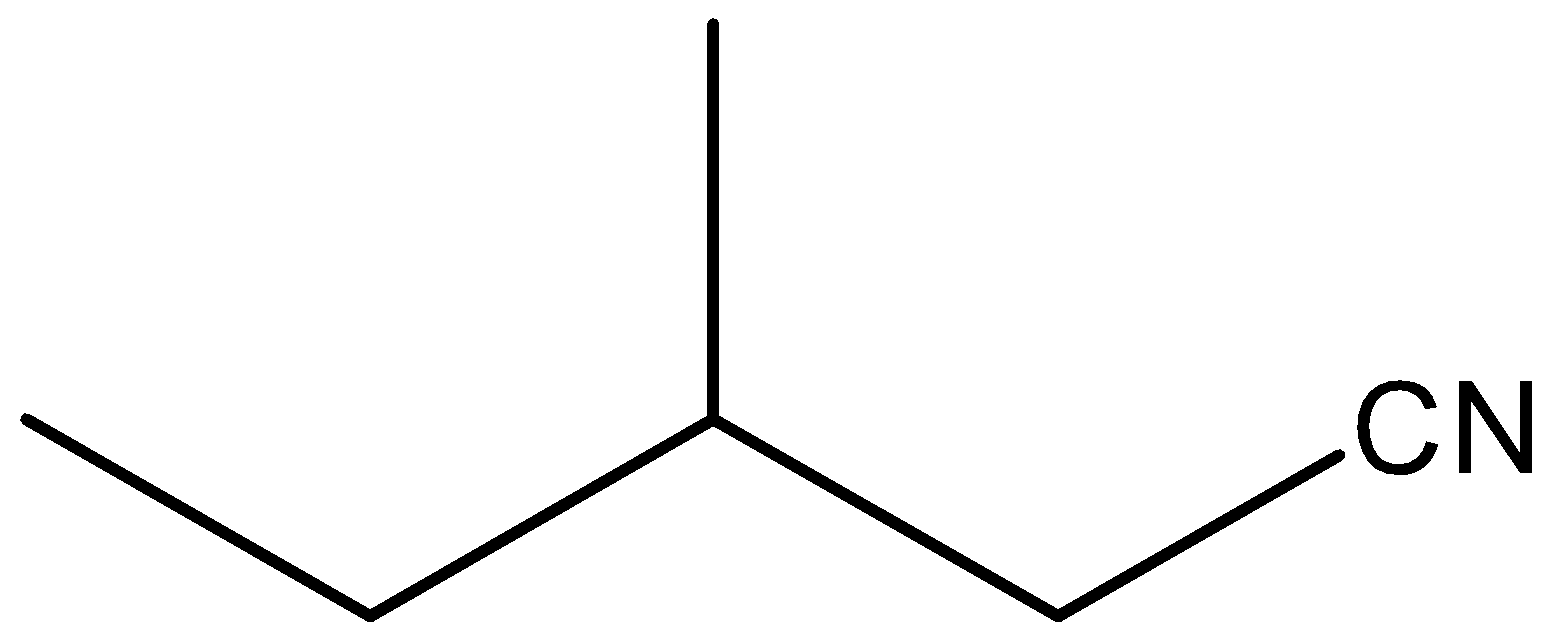
(C)
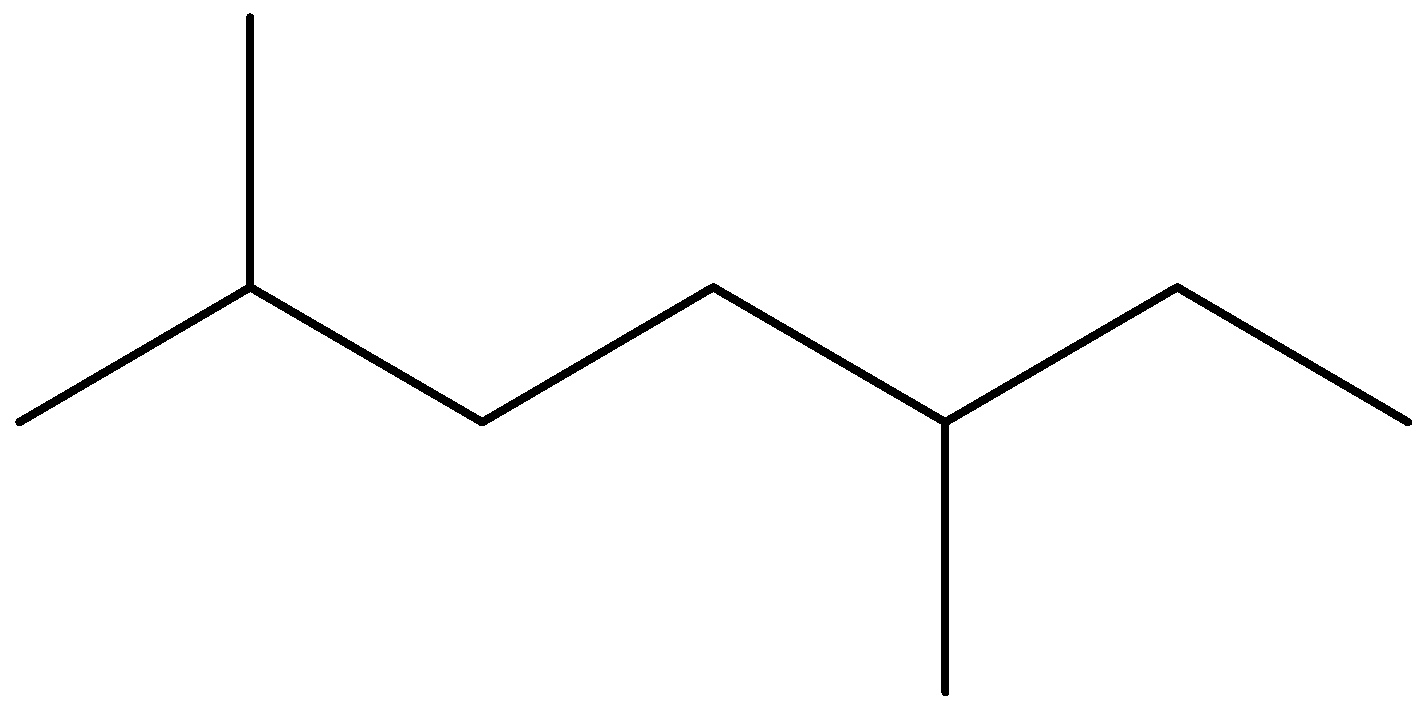
(D)
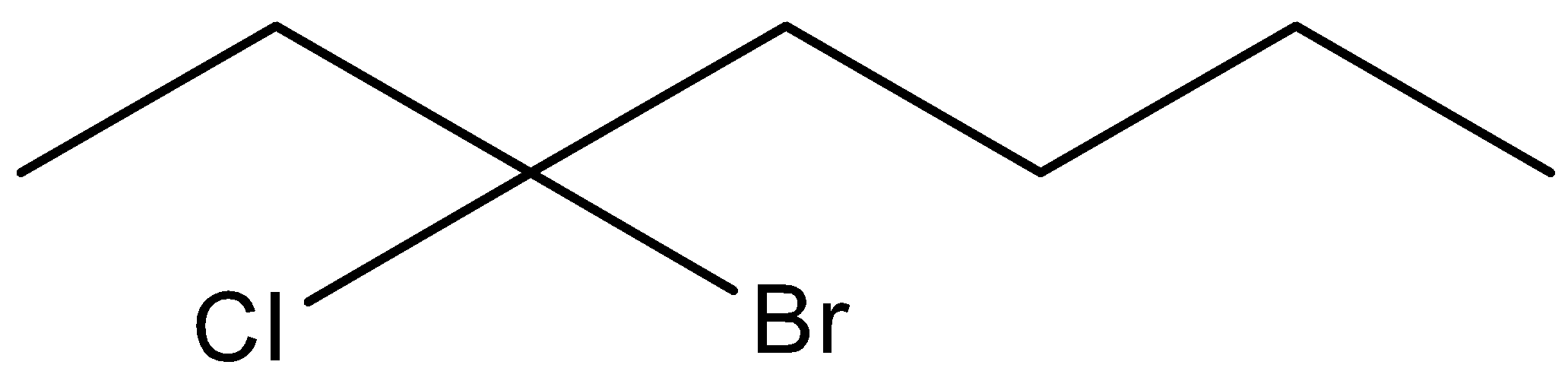
(E)
(F) $C{{l}_{2}}CHC{{H}_{2}}OH$





Answer
526.4k+ views
Hint:First try and identify the functional group present in the compound, then find the longest carbon chain present in the compound followed by finding out the substituent groups on the main chain and then using the suffix or prefix of functional group, you can name the compound by IUPAC method.
Complete step by step answer:
In order to give compounds a name, we must follow certain rules. When we want to name organic compounds, we should follow the IUPAC (International Union of Pure and Applied Chemistry) nomenclature (naming scheme).
We will try to know about the norms of IUPAC, that we should follow in naming organic compounds by answering the above question.
A.
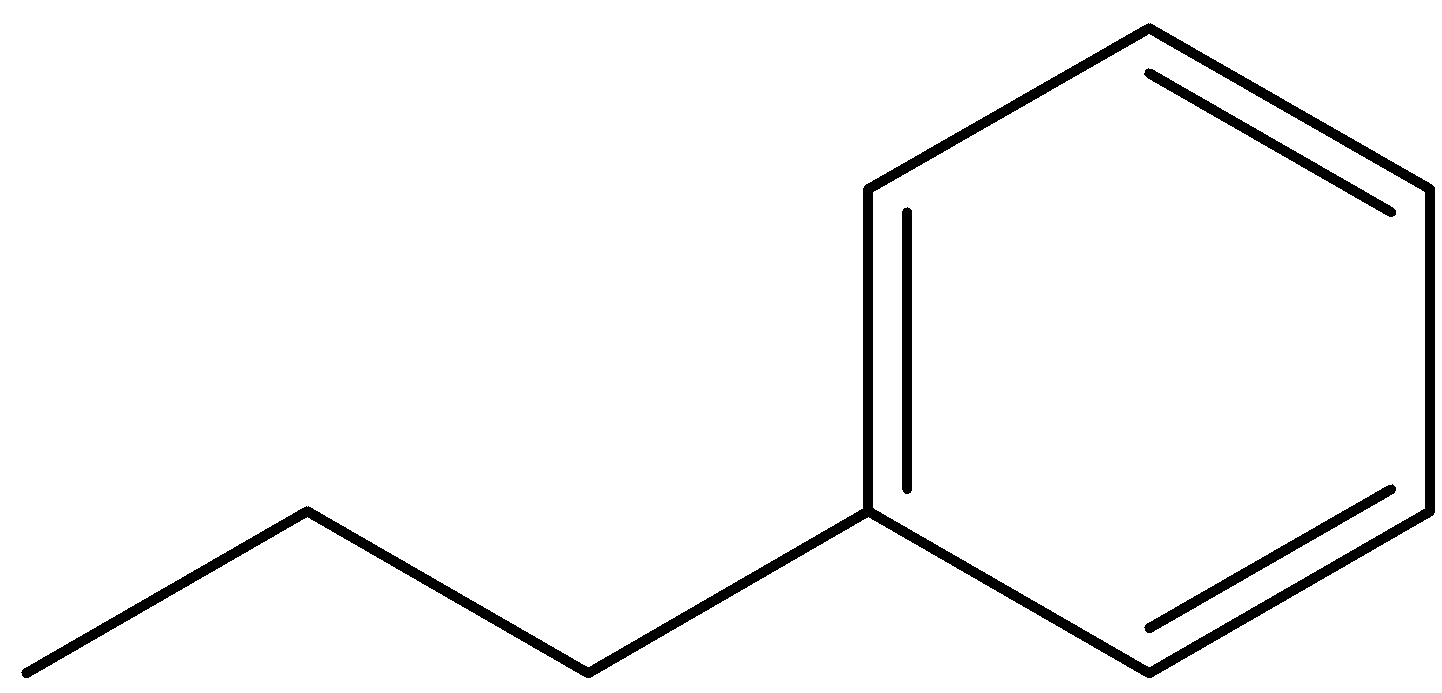
So, this is our first structure. Now, we can see that a carbon chain is attached to a phenyl ring. This carbon chain contains three carbons so it will be called propyl and this propyl group attached to a phenyl ring. So, its name will be Propyl-benzene or n-propyl benzene. Here, we have used n-propyl because the three carbons are in a line and the phenyl ring is substituted at the terminal carbon, hence n stands for normal propyl group.
B.
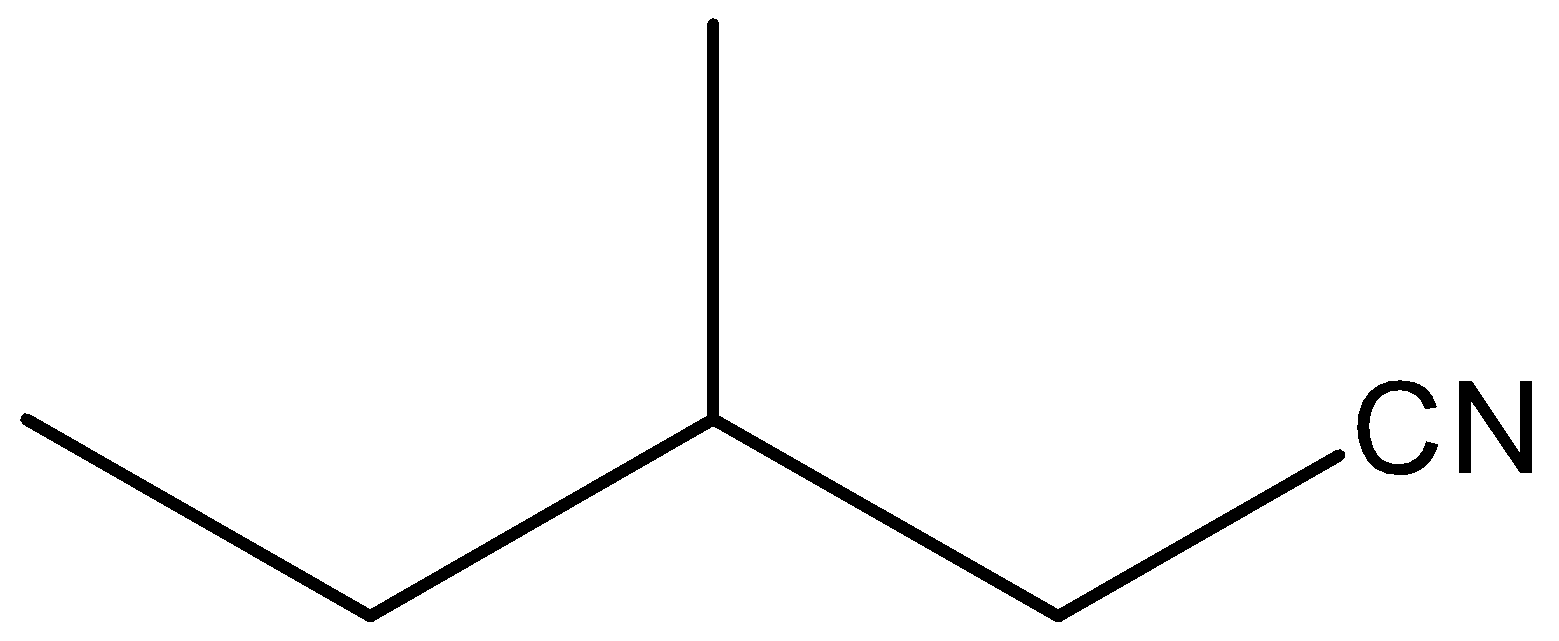
So, as we see in this structure a cyano functional group(C≡N) is attached. We should know that the suffix when naming a straight-chain alkanenitrile is always "nitrile". Here, we will also need to find the longest chain of carbon atoms which is of five carbons. Remember that we should start numbering the carbon chain in a way that the carbon bearing a functional group gets a minimum number. So, in this structure, a methyl group is attached at the third position of the chain. So, we have to name that also. So, from this discussion we can say that the IUPAC name of this structure is 3-methyl pentanenitrile.
C.
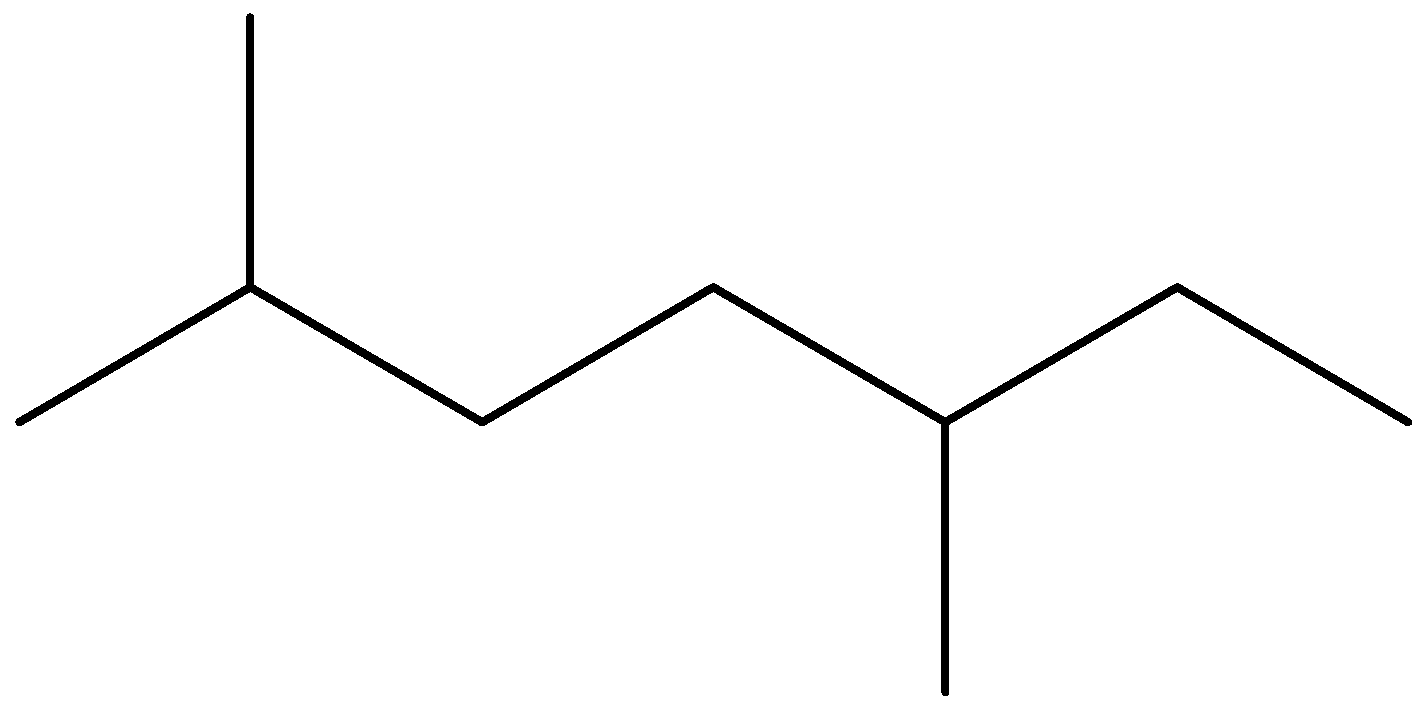
Now, we will name the third structure. To name this structure, we should first identify the longest chain and then we will identify the branches. We can see that seven carbon chains are possible here. There are two identical branches present at position second and fifth position of chain. We should note that we always give numbers from that carbon atom from the branches that have the lowest possible numbers. So, the IUPAC name of this compound is 2-5, dimethyl heptane.
Here in this compound, we can see that seven carbon chains are there. To give the IUPAC name of above structure, we should note that chloro and bromo group are attached at the third position of the chain. We should take third position because in this way the chloro and bromo group have lowest numbers possible. So, the IUPAC name of the above compound is 3− Bromo-3-chloro Heptane. Here, we have written bromo substitution first in the IUPAC name because they need to be arranged in an alphabetical order.
E.
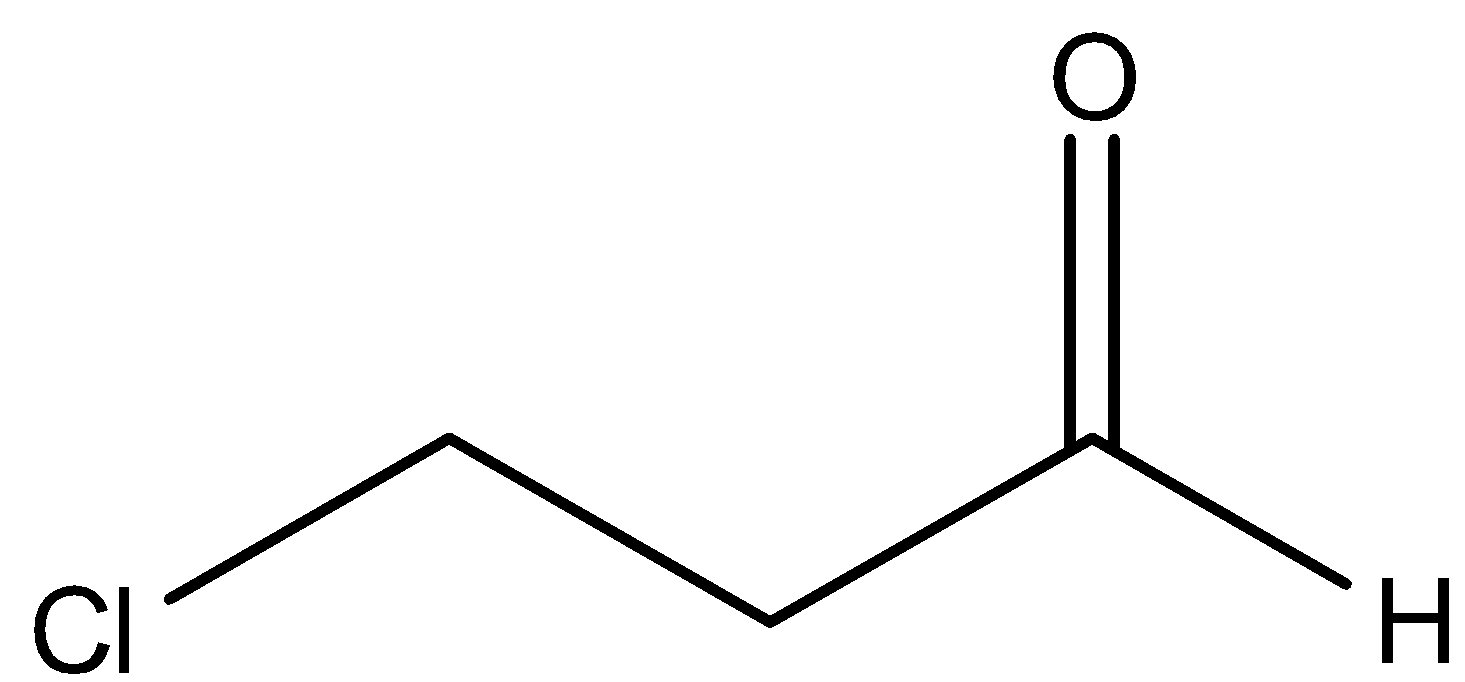
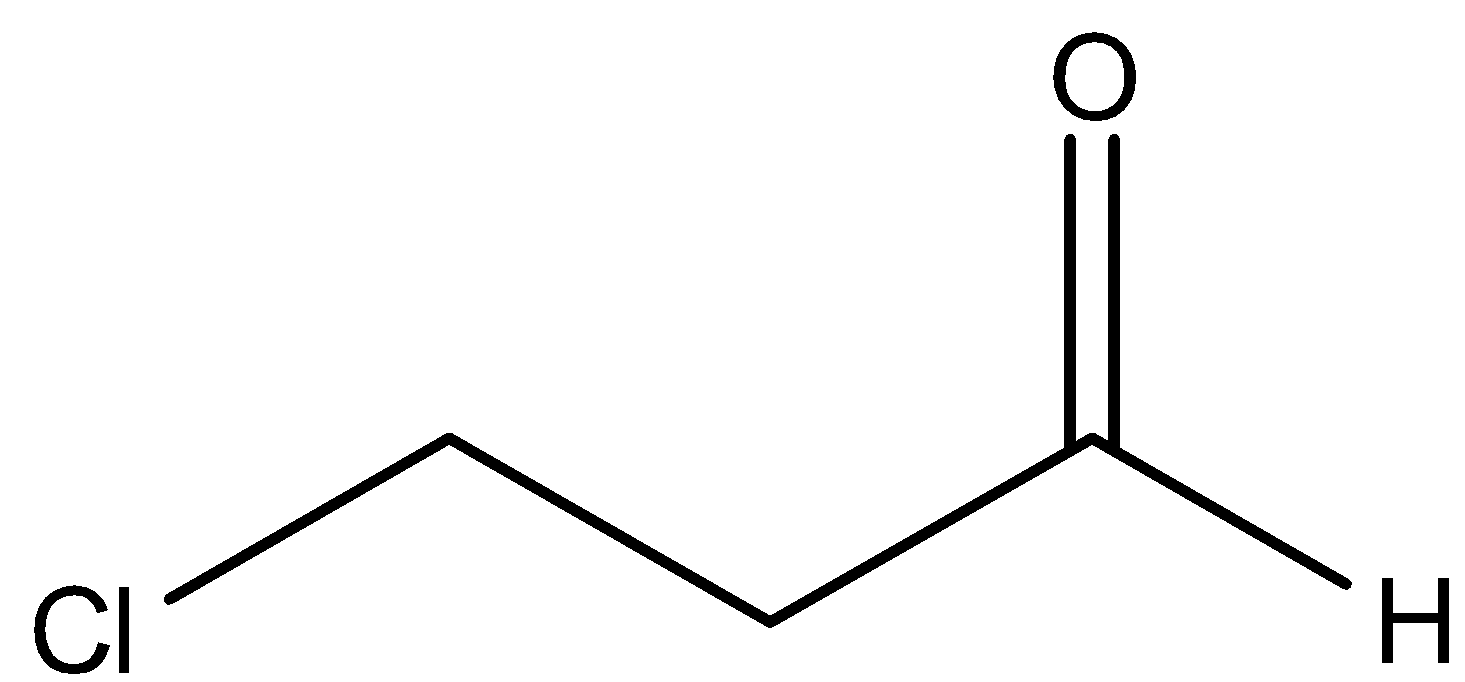
By observing the above structure, we came to know that there is one chlorine group attached at the end of the chain and oxygen is attached also. But it is not only oxygen, it is the aldehyde group because –CHO is there. Now, we can see that a three carbon chain is present here. We will start numbering from the carbon of the aldehyde group because it has more priority than the halogen group. So, we can say that there is a chlorine group attached at ${{3}^{rd}}$ carbon. There is an ‘-al’ suffix for aldehydes. So, we can name this compound as 3-Chloro propanal.
F. $C{{l}_{2}}CHC{{H}_{2}}OH$
As we see the above structure we came to know that the main functional group is alcohol. And two chloro groups are attached at second carbon. Because there is an alcohol group, so we should use the suffix –ol. Finally, we can say that the IUPAC name of the above structure is 2,2-Dichloroethanol.
Note:
If more than one type of substituent groups are present on the carbon chain then make sure that you write the IUPAC name of that compound in a way that all those substituent groups are arranged in an alphabetical manner. If a substituent group is present in the compound more than one time, then we should use Di-, Tri-, Tetra- prefix.
Complete step by step answer:
In order to give compounds a name, we must follow certain rules. When we want to name organic compounds, we should follow the IUPAC (International Union of Pure and Applied Chemistry) nomenclature (naming scheme).
We will try to know about the norms of IUPAC, that we should follow in naming organic compounds by answering the above question.
A.

So, this is our first structure. Now, we can see that a carbon chain is attached to a phenyl ring. This carbon chain contains three carbons so it will be called propyl and this propyl group attached to a phenyl ring. So, its name will be Propyl-benzene or n-propyl benzene. Here, we have used n-propyl because the three carbons are in a line and the phenyl ring is substituted at the terminal carbon, hence n stands for normal propyl group.
B.

So, as we see in this structure a cyano functional group(C≡N) is attached. We should know that the suffix when naming a straight-chain alkanenitrile is always "nitrile". Here, we will also need to find the longest chain of carbon atoms which is of five carbons. Remember that we should start numbering the carbon chain in a way that the carbon bearing a functional group gets a minimum number. So, in this structure, a methyl group is attached at the third position of the chain. So, we have to name that also. So, from this discussion we can say that the IUPAC name of this structure is 3-methyl pentanenitrile.
C.

Now, we will name the third structure. To name this structure, we should first identify the longest chain and then we will identify the branches. We can see that seven carbon chains are possible here. There are two identical branches present at position second and fifth position of chain. We should note that we always give numbers from that carbon atom from the branches that have the lowest possible numbers. So, the IUPAC name of this compound is 2-5, dimethyl heptane.
D.
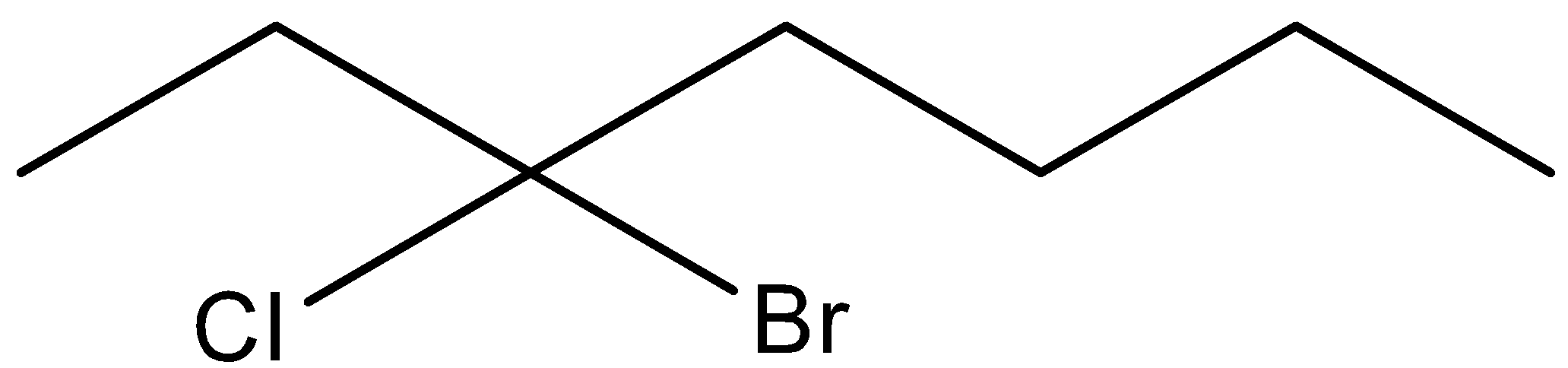

Here in this compound, we can see that seven carbon chains are there. To give the IUPAC name of above structure, we should note that chloro and bromo group are attached at the third position of the chain. We should take third position because in this way the chloro and bromo group have lowest numbers possible. So, the IUPAC name of the above compound is 3− Bromo-3-chloro Heptane. Here, we have written bromo substitution first in the IUPAC name because they need to be arranged in an alphabetical order.
E.

By observing the above structure, we came to know that there is one chlorine group attached at the end of the chain and oxygen is attached also. But it is not only oxygen, it is the aldehyde group because –CHO is there. Now, we can see that a three carbon chain is present here. We will start numbering from the carbon of the aldehyde group because it has more priority than the halogen group. So, we can say that there is a chlorine group attached at ${{3}^{rd}}$ carbon. There is an ‘-al’ suffix for aldehydes. So, we can name this compound as 3-Chloro propanal.
F. $C{{l}_{2}}CHC{{H}_{2}}OH$
As we see the above structure we came to know that the main functional group is alcohol. And two chloro groups are attached at second carbon. Because there is an alcohol group, so we should use the suffix –ol. Finally, we can say that the IUPAC name of the above structure is 2,2-Dichloroethanol.
Note:
If more than one type of substituent groups are present on the carbon chain then make sure that you write the IUPAC name of that compound in a way that all those substituent groups are arranged in an alphabetical manner. If a substituent group is present in the compound more than one time, then we should use Di-, Tri-, Tetra- prefix.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




