
Give one chemical test to distinguish between ethylamine and aniline.
Answer
534.9k+ views
Hint: The products of the reaction of nitrous acid with aliphatic and aromatic amines show different chemical behaviours. Products from aliphatic amines give effervescence of nitrogen whereas products from aromatic amines form bright colored dyes. Ethylamine is aliphatic amine and aniline is aromatic amine.
Complete answer:
When aromatic amines react with nitrous acid at low temperature, diazonium salt is formed. Then coupling the diazonium salt with 2-naphthol in presence of base such as aqueous sodium hydroxide solution. We will get a bright coloured azo dye. But if we add nitrous acid to aliphatic amines, then effervescence of nitrogen gas is obtained.
Thus, we can distinguish between ethylamine and aniline by using azo dye test.
Aniline is an aromatic amine and ethyl amine is aliphatic amine. Aniline reacts with nitrous acid at low temperature; it forms benzene diazonium chloride (a diazo salt). Nitrous acid is prepared in situ by the reaction of sodium nitrite on dilute hydrochloric acid.
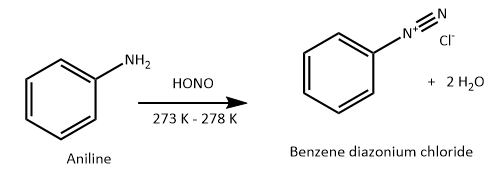
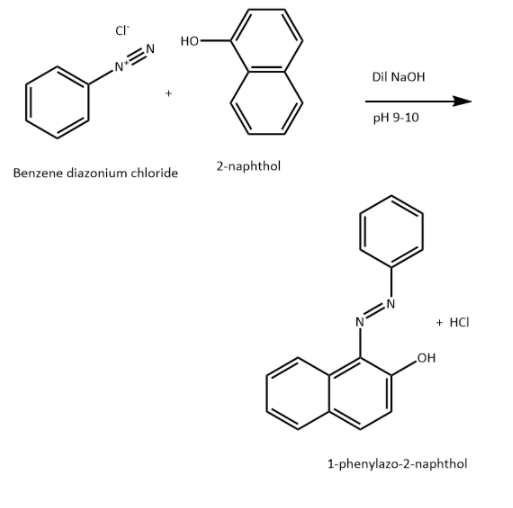
Benzene diazonium chloride then reacts with an alkaline solution of 2-naphthol to form orange dye.
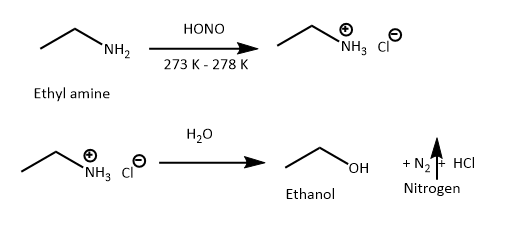
However when ethyl amine is subjected to similar conditions, brisk effervescence of nitrogen gas are observed.
So Azo dye test is used to differentiate between ethylamine and aniline.
Note: Azo dyes are a wide variety (around 2000 types) of synthetic dyes that contain C - N = N -C linkage. Azo dyes are used to impart color to leather, textiles, articles and some foods. Azo dyes are low cost, easy to apply, and have strong, clear colors. Thus, azo dyes have wide applications in the dye industry.
Complete answer:
When aromatic amines react with nitrous acid at low temperature, diazonium salt is formed. Then coupling the diazonium salt with 2-naphthol in presence of base such as aqueous sodium hydroxide solution. We will get a bright coloured azo dye. But if we add nitrous acid to aliphatic amines, then effervescence of nitrogen gas is obtained.
Thus, we can distinguish between ethylamine and aniline by using azo dye test.
Aniline is an aromatic amine and ethyl amine is aliphatic amine. Aniline reacts with nitrous acid at low temperature; it forms benzene diazonium chloride (a diazo salt). Nitrous acid is prepared in situ by the reaction of sodium nitrite on dilute hydrochloric acid.

Benzene diazonium chloride then reacts with an alkaline solution of 2-naphthol to form orange dye.

However when ethyl amine is subjected to similar conditions, brisk effervescence of nitrogen gas are observed.

So Azo dye test is used to differentiate between ethylamine and aniline.
Note: Azo dyes are a wide variety (around 2000 types) of synthetic dyes that contain C - N = N -C linkage. Azo dyes are used to impart color to leather, textiles, articles and some foods. Azo dyes are low cost, easy to apply, and have strong, clear colors. Thus, azo dyes have wide applications in the dye industry.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




