
Give IUPAC names of the following ethers:
(i)
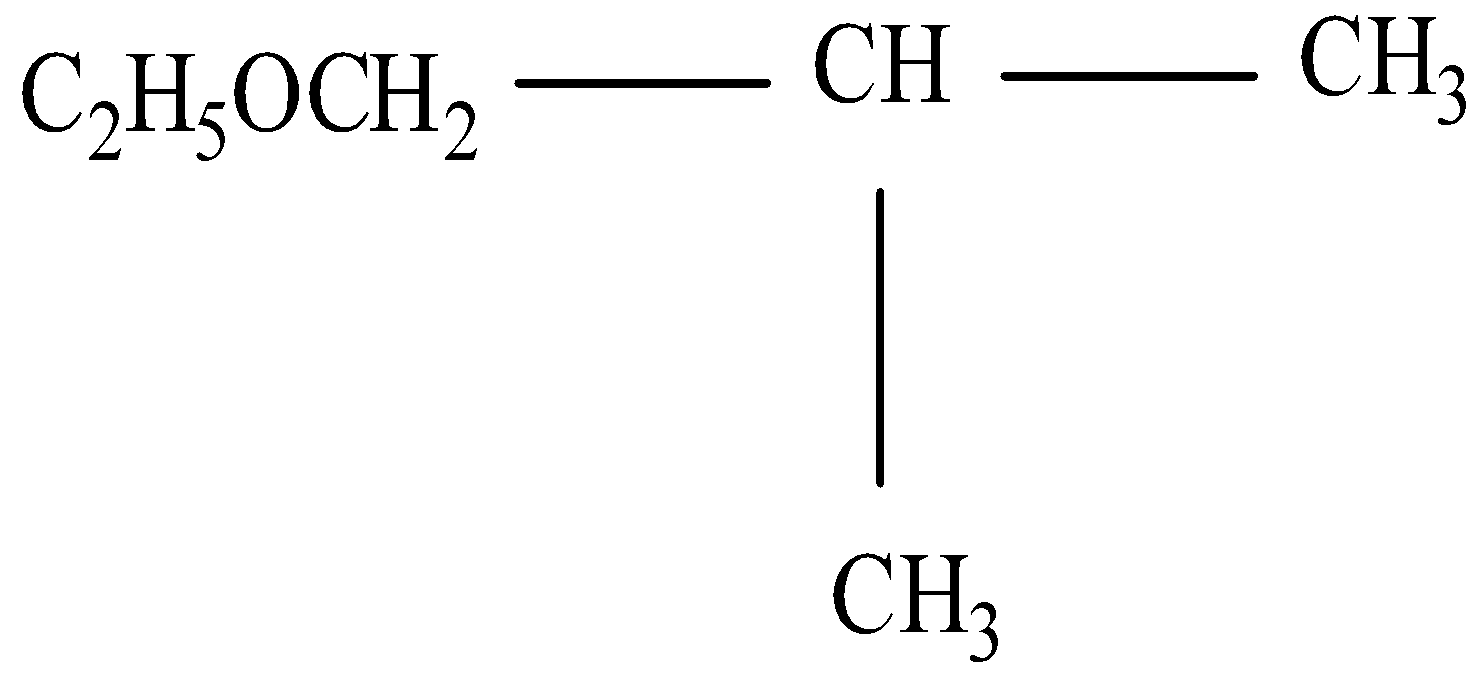
(ii)
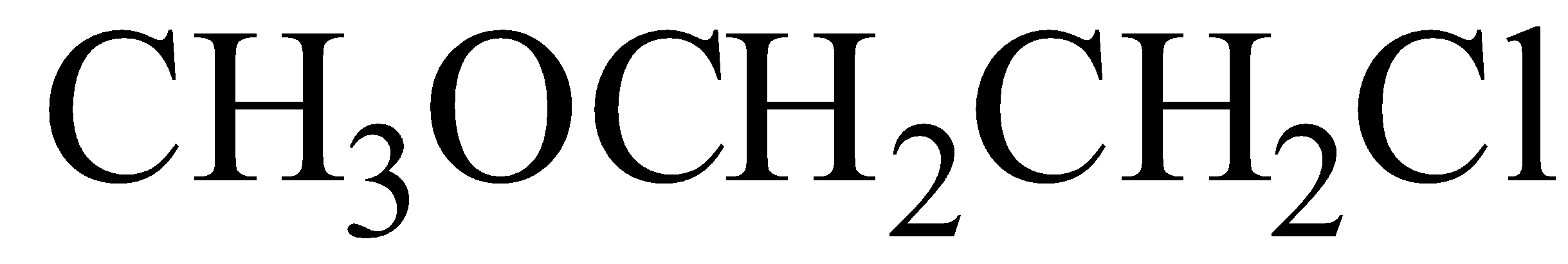
(iii)
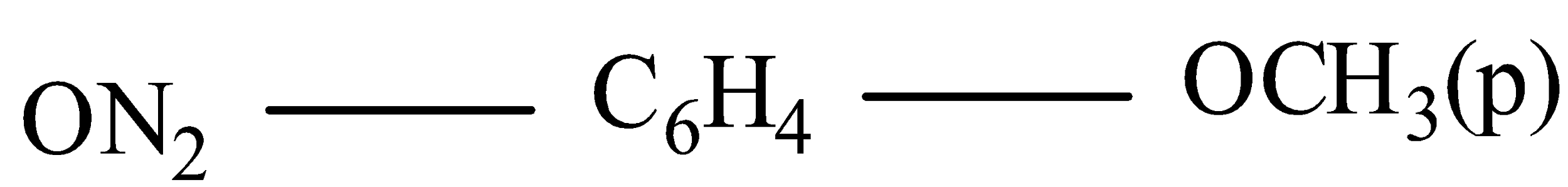
(iv)
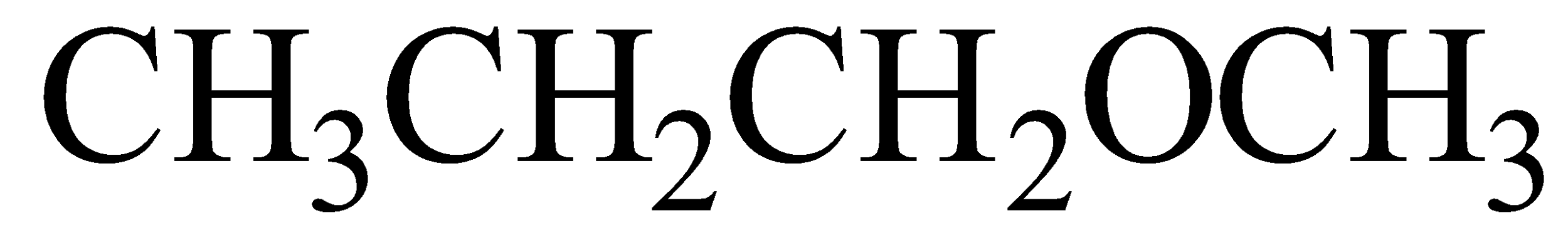
(v)
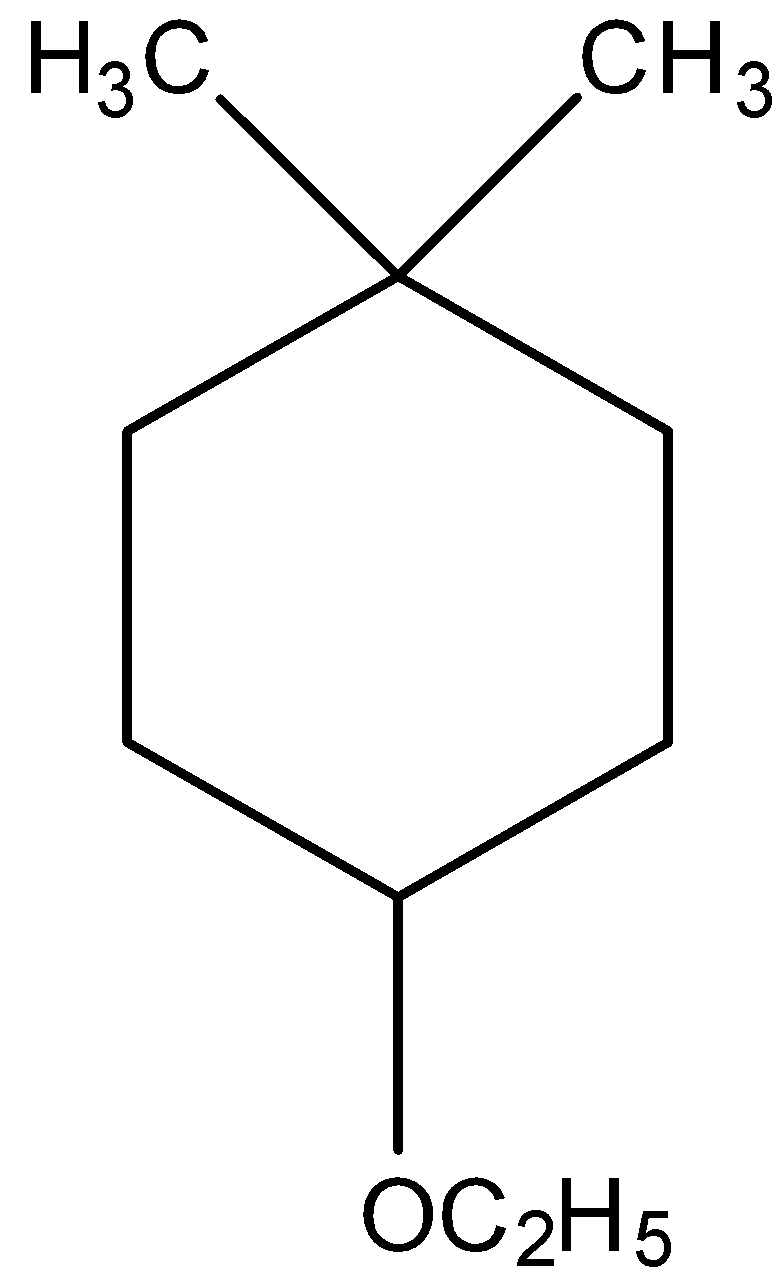
(vi)
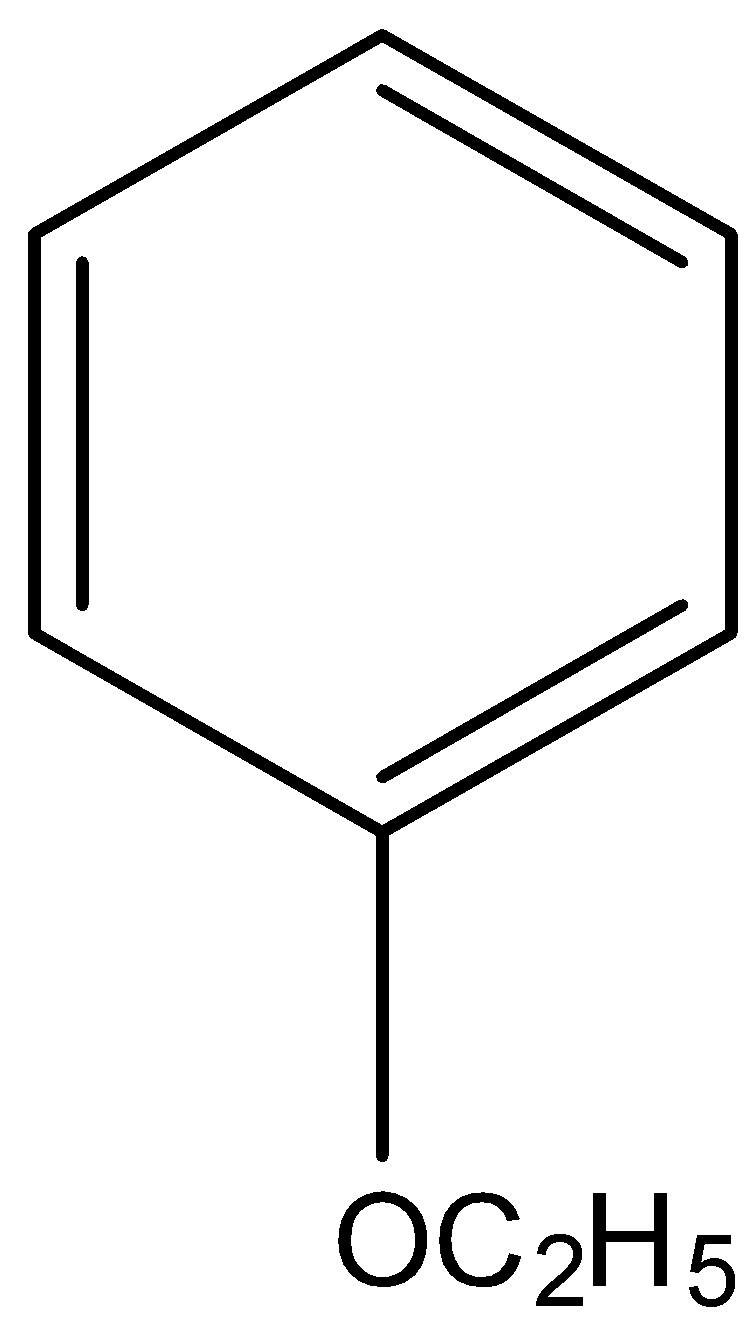

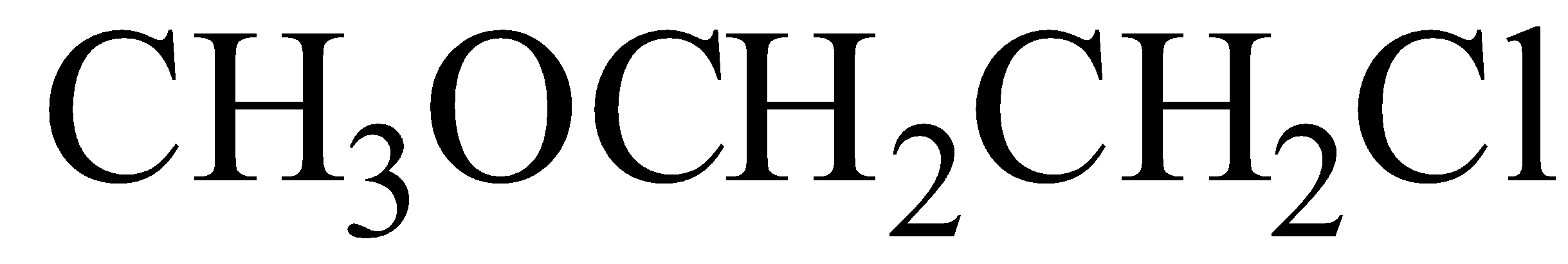
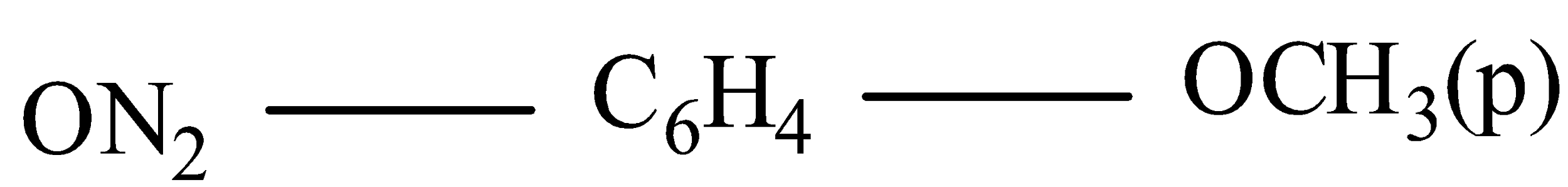
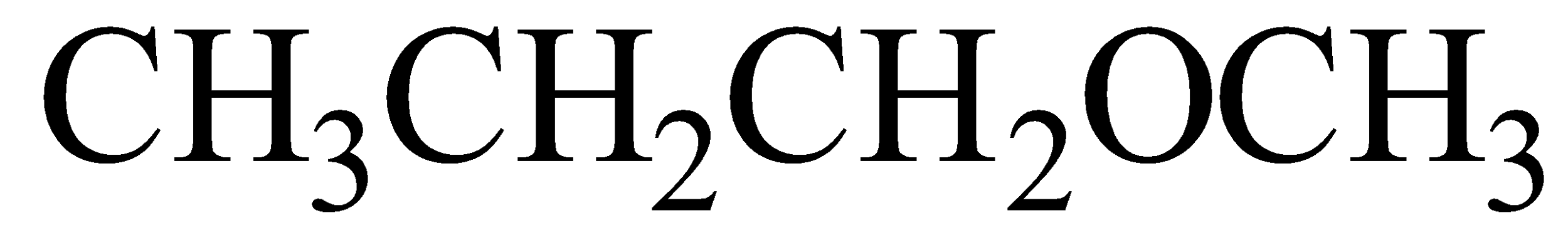


Answer
588.6k+ views
Hint: To write the IUPAC name of ether, firstly find the parent atom and substituent group in the compound on either side of the oxygen. Substituent groups will contain less no of carbon atoms and be named with a prefix of oxy. Firstly write the name of the substituent group and then the name of the parent atom.
Complete step by step solution:
Ethers are the class of organic compound containing an oxygen atom bonded with two different or same
aryl or alkyl group. General formula of ether is, $R-O-R$ where O represents the oxygen group and R represents an alkyl group.
Steps to do the nomenclature of ether
(1) First step is to find which alkyl or aryl group is attached with the oxygen atom.
(2) Then determine which one is a parental atom and which one is a substituent group among them. Substituent groups have shorter alkyl groups and will be named with a prefix oxy and the larger alkyl group will be the parental atom.
(3) And if the same alkyl group is attached on both the side then it should be written as 'di'
(4) Carbon closest to the oxygen will get the priority in numbering.
(5) write the name alkoxy group first and then the parent atom should be written. And if there is another substituent group also present then write them according to the alphabet priority rule.
Now let us do the nomenclature of all the compounds given in the question one by one.
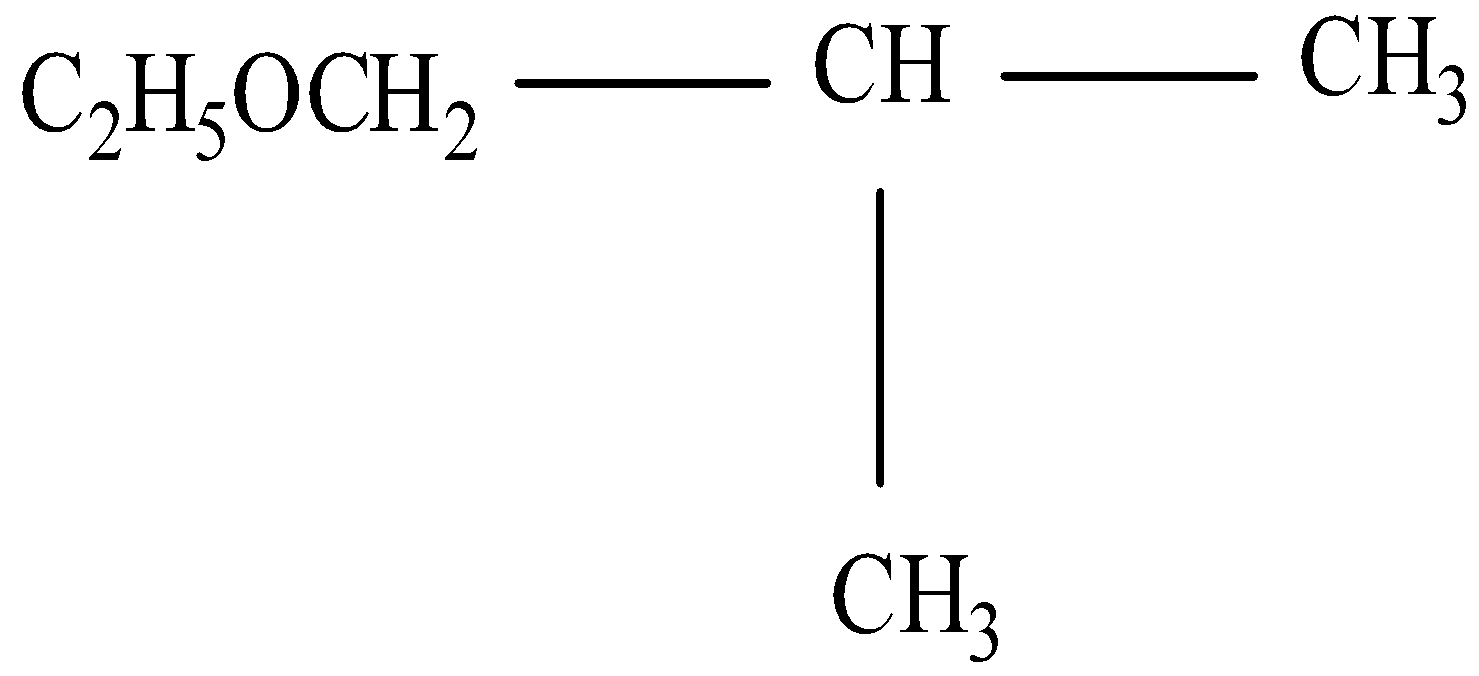
(i)
Firstly we will do the numbering of carbon,
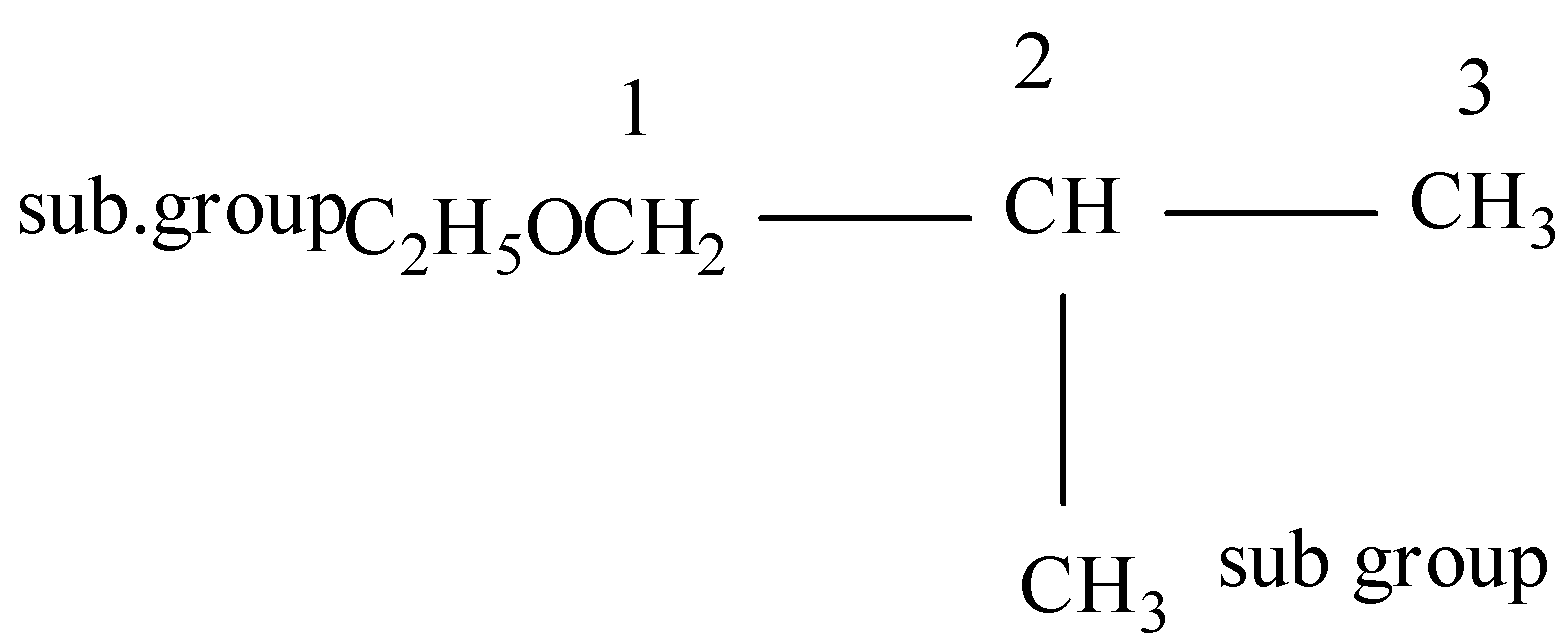
So, here there are two substituent groups, one in the ethoxy group and another is methyl so the methoxy will be written first as per the alphabet rule and then the methyl and after that the parent atom should be written.
Thus the IUPAC will be 1-Ethoxy-2-methylpropane.
(ii)
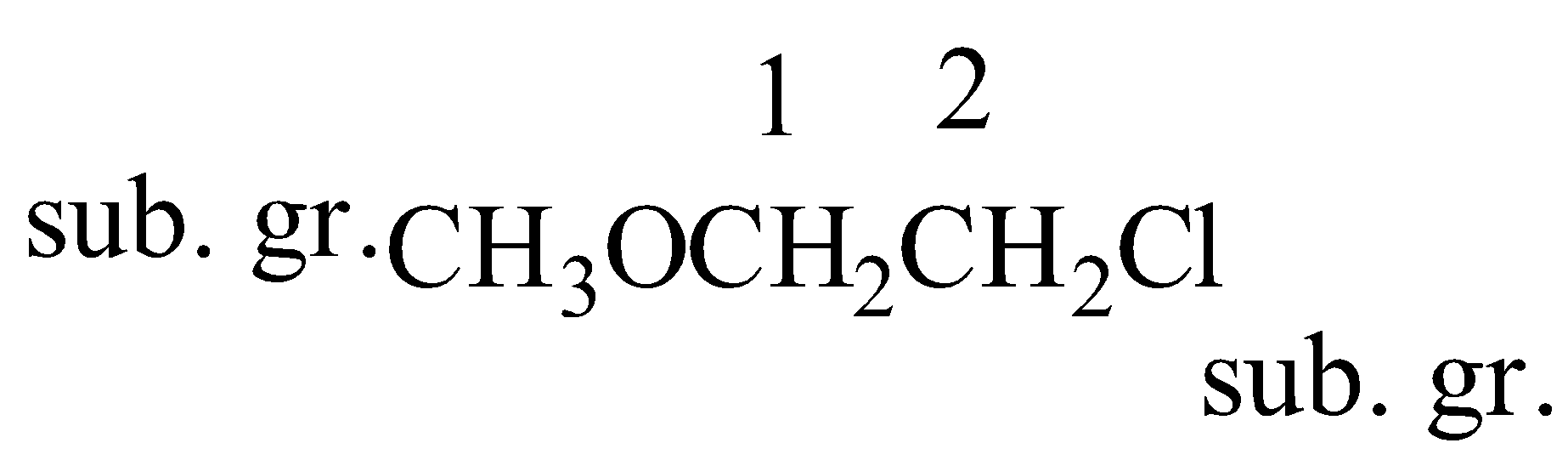
IUPAC name of this compound is 2-Chloro-1-methoxyethane(here c comes first in the alphabet than m so chlorine is written first)
(iii)
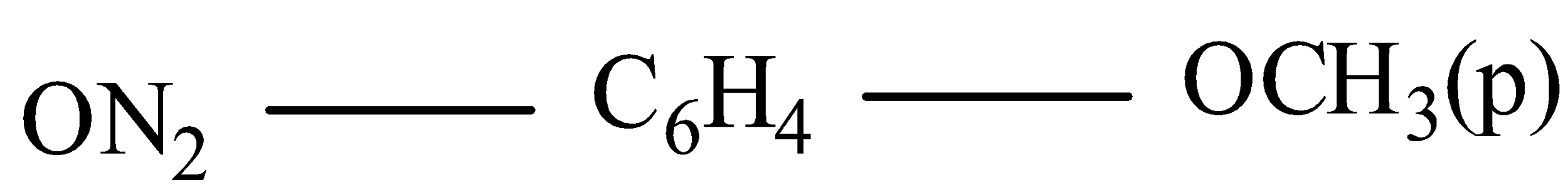
IUPAC name 4-Nitroanisole( here methoxy group is attached with benzene group it is written as anisole).
(iv)
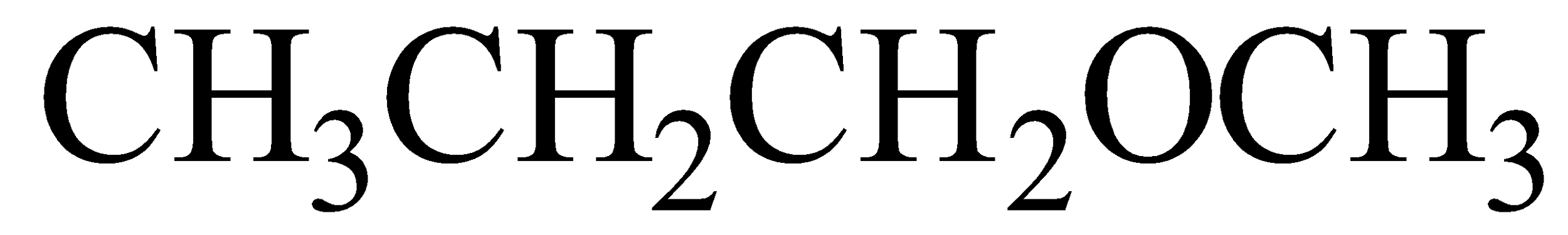
IUPAC name 1-Methoxy propane
(v)
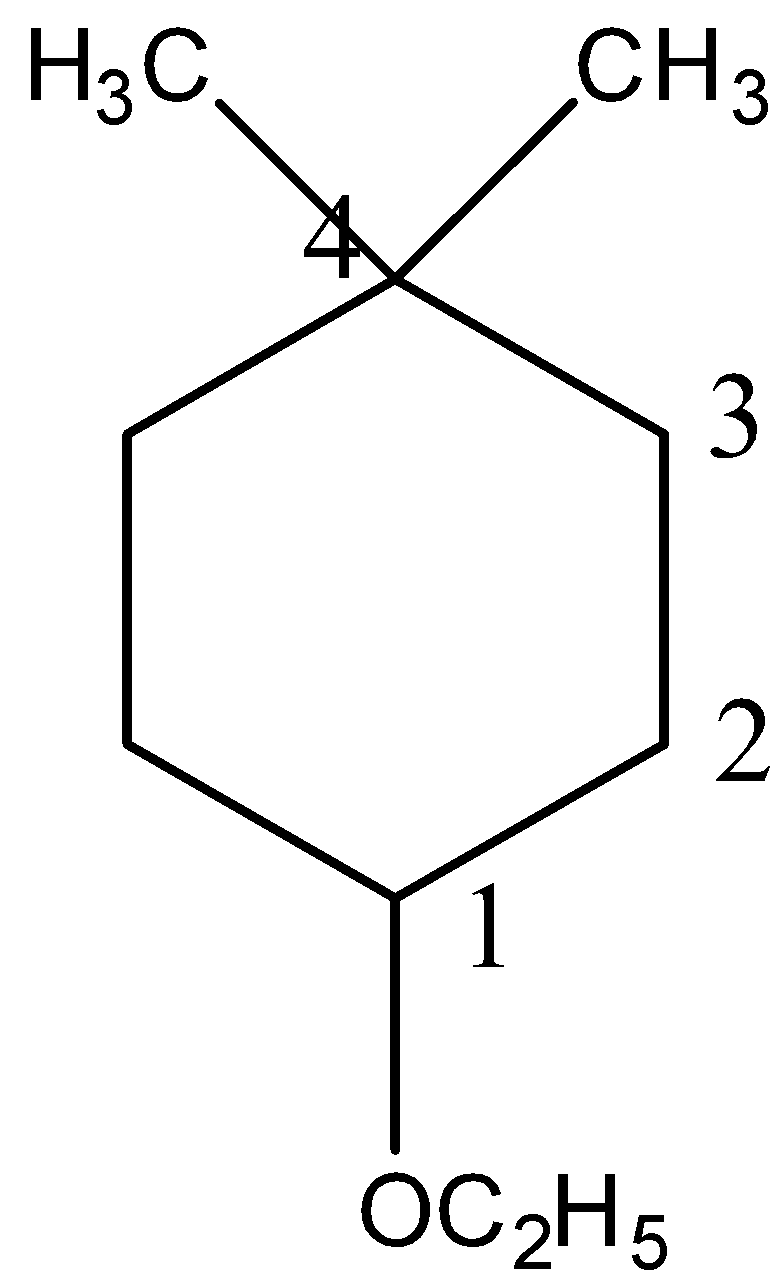
IUPAC name will be 1-Ethoxy-4,4-dimethylcyclohexane.
(vi)
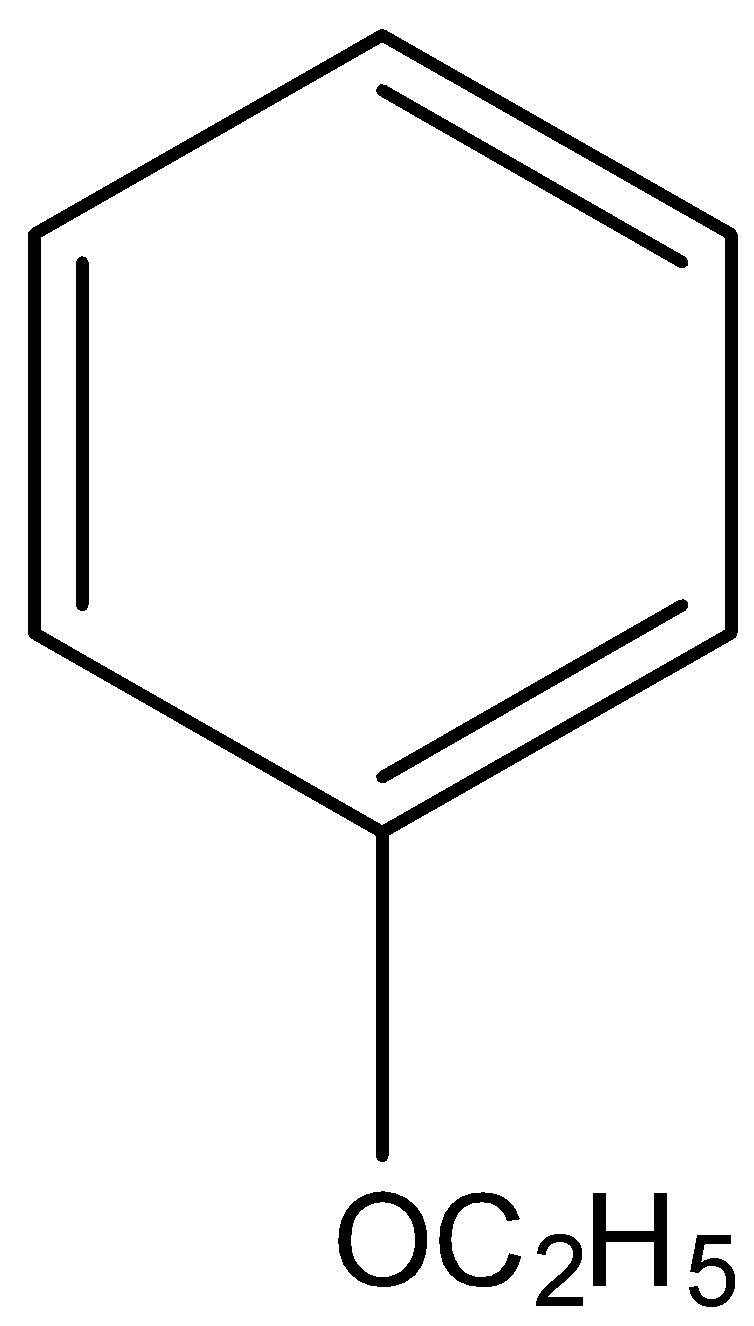
IUPAC name Ethoxybenzene.
Note: Substituent should be written first and not the parent group. And if there is any alicyclic( cyclo compound with no double bond) structure Cyclo is must to write with the parent atom. If there is more than one substituent then apply the alphabet priority rule. In the presence of double or triple bond numbering should be done from that carbon which is attached with double bond.
Complete step by step solution:
Ethers are the class of organic compound containing an oxygen atom bonded with two different or same
aryl or alkyl group. General formula of ether is, $R-O-R$ where O represents the oxygen group and R represents an alkyl group.
Steps to do the nomenclature of ether
(1) First step is to find which alkyl or aryl group is attached with the oxygen atom.
(2) Then determine which one is a parental atom and which one is a substituent group among them. Substituent groups have shorter alkyl groups and will be named with a prefix oxy and the larger alkyl group will be the parental atom.
(3) And if the same alkyl group is attached on both the side then it should be written as 'di'
(4) Carbon closest to the oxygen will get the priority in numbering.
(5) write the name alkoxy group first and then the parent atom should be written. And if there is another substituent group also present then write them according to the alphabet priority rule.
Now let us do the nomenclature of all the compounds given in the question one by one.
(i)

Firstly we will do the numbering of carbon,
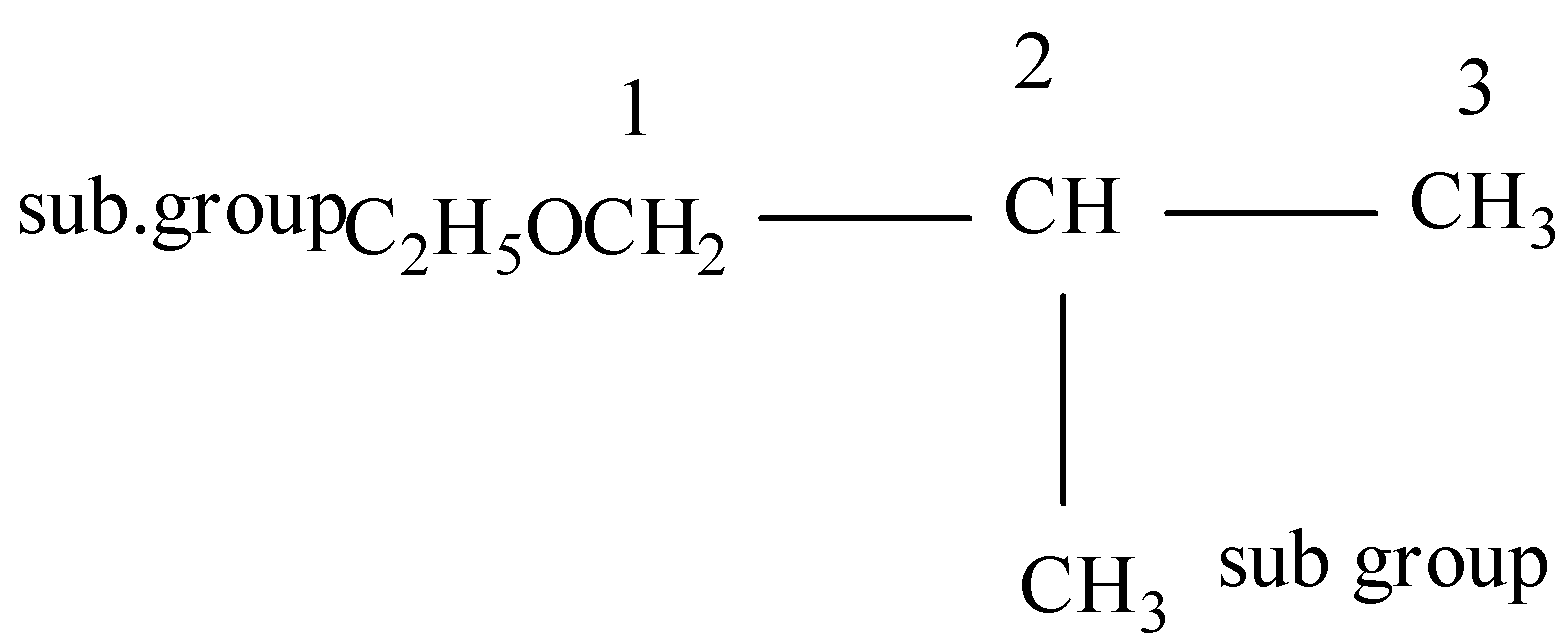
So, here there are two substituent groups, one in the ethoxy group and another is methyl so the methoxy will be written first as per the alphabet rule and then the methyl and after that the parent atom should be written.
Thus the IUPAC will be 1-Ethoxy-2-methylpropane.
(ii)
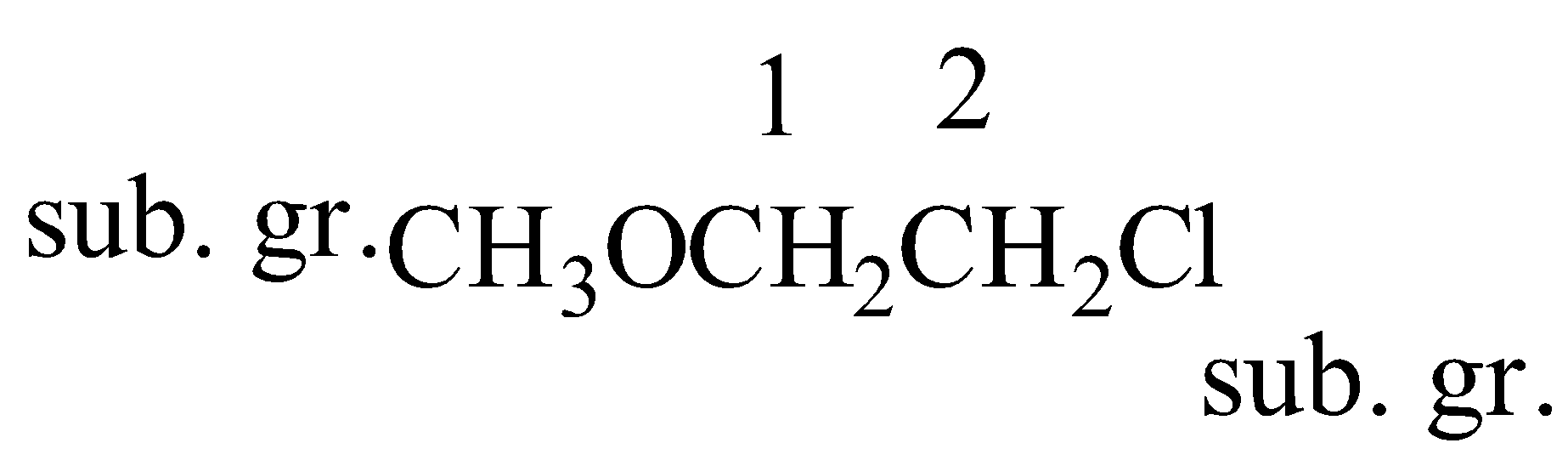
IUPAC name of this compound is 2-Chloro-1-methoxyethane(here c comes first in the alphabet than m so chlorine is written first)
(iii)
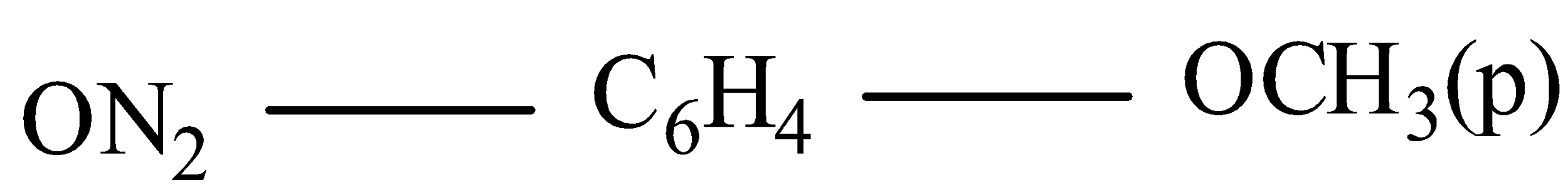
IUPAC name 4-Nitroanisole( here methoxy group is attached with benzene group it is written as anisole).
(iv)
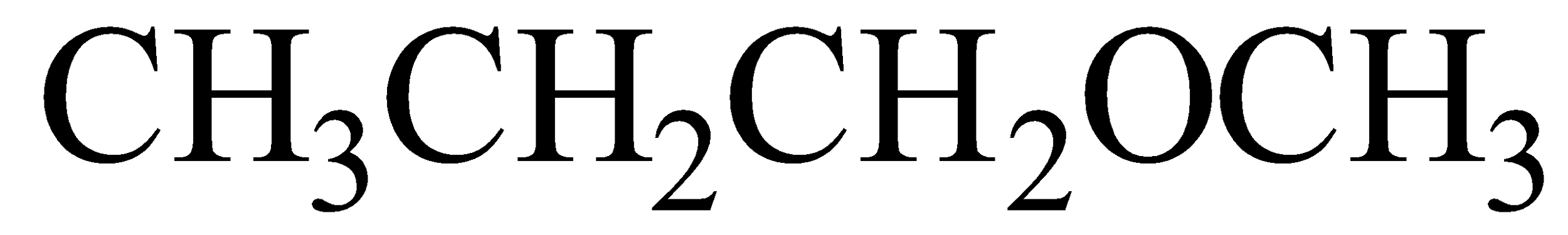
IUPAC name 1-Methoxy propane
(v)

IUPAC name will be 1-Ethoxy-4,4-dimethylcyclohexane.
(vi)

IUPAC name Ethoxybenzene.
Note: Substituent should be written first and not the parent group. And if there is any alicyclic( cyclo compound with no double bond) structure Cyclo is must to write with the parent atom. If there is more than one substituent then apply the alphabet priority rule. In the presence of double or triple bond numbering should be done from that carbon which is attached with double bond.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




