
What is the geometry of cis-diamminedichloroplatinum (II)?
Answer
489.9k+ views
Hint: Diamminedichloroplatinum is a neutral complex, it can exist in two forms. Cis-diamminedichloroplatinum (II) which is known as cisplatin which is used to cure cancer and trans- diamminedichloroplatinum (II) known as transplatin.
Complete Step By Step Answer:
For a four-coordinate metal-ligand complex, we can have either a tetrahedral, square planar, or see-saw geometry. The given complex is cis-diamminedichloroplatinum (II), cis means same side, therefore two identical ligands will be ${90^\circ }$ apart for the cis configuration.
It is a planar molecule. The complex is a neutral complex because platinum will give $ + 2$ charge to the complex, chlorine will give $ - 2$ charge to the complex as there are two chlorine atoms and ammonia will contribute zero charge, thus the complex is neutral.
In the diamminedichloroplatinum (II) complex, platinum will be the central metal atom. It has four ligands surrounding it, which are two chlorine atoms and two ammonia atoms the cis-isomer have the structure as:
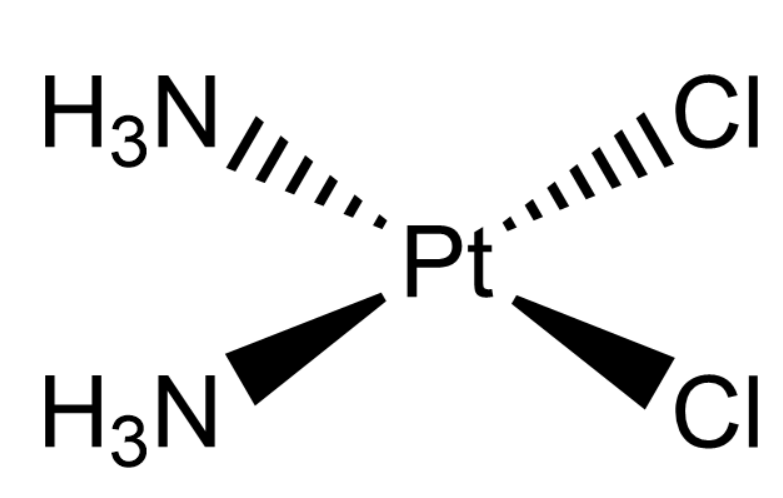
The tetrahedral and see-saw geometry do not have cis or Trans complexes thus, we can predict that the cis- diamminedichloroplatinum (II) has a square planar geometry.
Note:
When two chlorine are adjacent and two ammonia atom s are adjacent to each other they form a cis isomer known as cisplatin it can be used as a medication to treat various cancers such as balder cancer, breast cancer etc. whereas when two identical ligands are placed opposite to each other the isomer is known as trans isomers and it does not have much applications as cisplatin.
Complete Step By Step Answer:
For a four-coordinate metal-ligand complex, we can have either a tetrahedral, square planar, or see-saw geometry. The given complex is cis-diamminedichloroplatinum (II), cis means same side, therefore two identical ligands will be ${90^\circ }$ apart for the cis configuration.
It is a planar molecule. The complex is a neutral complex because platinum will give $ + 2$ charge to the complex, chlorine will give $ - 2$ charge to the complex as there are two chlorine atoms and ammonia will contribute zero charge, thus the complex is neutral.
In the diamminedichloroplatinum (II) complex, platinum will be the central metal atom. It has four ligands surrounding it, which are two chlorine atoms and two ammonia atoms the cis-isomer have the structure as:

The tetrahedral and see-saw geometry do not have cis or Trans complexes thus, we can predict that the cis- diamminedichloroplatinum (II) has a square planar geometry.
Note:
When two chlorine are adjacent and two ammonia atom s are adjacent to each other they form a cis isomer known as cisplatin it can be used as a medication to treat various cancers such as balder cancer, breast cancer etc. whereas when two identical ligands are placed opposite to each other the isomer is known as trans isomers and it does not have much applications as cisplatin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




